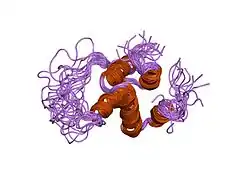
NLRP1
NLRP1 encodes NACHT, LRR, FIIND, CARD domain and PYD domains-containing protein 1 in humans.[5][6][7] NLRP1 was the first protein shown to form an inflammasome.[8] NLRP1 is expressed by a variety of cell types, which are predominantly epithelial or hematopoietic. The expression is also seen within glandular epithelial structures including the lining of the small intestine, stomach, airway epithelia and in hairless or glabrous skin.[9] NLRP1 polymorphisms are associated with skin extra-intestinal manifestations in CD.[9] Its highest expression was detected in human skin, in psoriasis and in vitiligo. Polymorphisms of NLRP1 were found in lupus erythematosus and diabetes type 1.[10] Variants of mouse NLRP1 were found to be activated upon N-terminal cleavage by the protease in anthrax lethal factor.[8]
Function
This gene encodes a member of the Ced-4 family of apoptosis proteins. Ced-family members contain a caspase recruitment domain (CARD) and are known to be key mediators of programmed cell death. The encoded protein contains a distinct N-terminal pyrin-like motif, which is possibly involved in protein-protein interactions. The NLRP1 protein interacts strongly with caspase 2 and weakly with caspase 9. Overexpression of this gene was demonstrated to induce pyroptosis in cells. Multiple alternatively spliced transcript variants encoding distinct isoforms have been found for this gene, but the biological validity of some variants has not been determined.[7]
Mechanism of activation
NLRP1 activates an antibacterial or antiviral immune response. Antibacterial immune response compensates for the loss of the MAP kinase response. Humans produce NLRP1, but human NLRP1 is not activated by lethal factor.[8] NLRP1 could be activated by proteolytic cleavage, resulting in the removal of an auto-inhibitory PYD and release of the CARD domain, responsible for the recruitment and activation of pro-caspase-1 in the active form of caspase-1.[8] Human NLRP1 activation can be elicited by several means including enteroviral 3C proteases.[11] Its function in immunity is just beginning to be understood.[8]
Interactions
NLRP1 has been shown to interact with caspase 9[12][13] and APAF1.[12] Via its FIIND domain, NLRP1 interacts directly with DPP9 and DPP8 which are needed to prevent NLRP1 activation.[14]
Loss of DPP9 in humans and mice, results in NLRP1 activation.[15]
Variants of NLRP1 in human
As published by Bruno Reversade and colleagues, several Mendelian diseases caused by NLRP1 germline mutations have been described.[16] These include Multiple Self-healing Palmoplantar Carcinoma, familial Nikam's disease and Autoinflammation with Arthritis and Dyskeratosis. Mutations in NLRP1, whether dominant or recessive, tend to be gain-of-function alleles that trigger inflammasome signaling with IL1B and IL18 release.
Variants of NLRP1 in mice
Mice have three paralogs of the Nlrp1 gene (Nlrp1a, b, c). Nlrp1c is a pseudogene.[17] Mouse NLRP1B is not activated by a receptor-ligand type mechanism. NLRP1B variants from certain inbred mouse strains, BALB/c and 129, can be activated by the lethal factor (LF) protease. The lethal factor protease is produced and secreted by Bacillus anthracis, the agent of anthrax.[18] Together with protective antigen (PA), LF forms a bipartite toxin, Lethal Toxin. The role of PA is to form a translocation channel that delivers LF into the host cell cytosol, where LF play roles in immune response by cleaving and inactivating MAP kinases.[19][20] LF also directly cleaves NLRP1B proximal to its N-terminus, it is necessary and sufficient for NLRP1B inflammasome formation and CASP1 activation.[21] Activation of NLRP1B-dependent inflammasome responses appears in host defense with mechanism like IL-1β and neutrophils.[22][23] NLRP1B can function as a sensor of bacterial proteases, immune responses are specifically activated by virulence factors.[24][25]
It is not clear what stimuli might activate NLRP1A, the other known functional murine NLRP1 paralog. The study identified a mouse carrying a missense gain-of-function mutation in NLRP1A (Q593P) that active inflammasome responses. The mechanism of wild-type NLRP1A activation is unclear.[26]
References
- GRCh38: Ensembl release 89: ENSG00000091592 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000070390 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Thorpe KL, Abdulla S, Kaufman J, Trowsdale J, Beck S (Oct 1996). "Phylogeny and structure of the RING3 gene". Immunogenetics. 44 (5): 391–6. doi:10.1007/BF02602785. PMID 8781126. S2CID 44613743.
- Tschopp J, Martinon F, Burns K (February 2003). "NALPs: a novel protein family involved in inflammation". Nature Reviews. Molecular Cell Biology. 4 (2): 95–104. doi:10.1038/nrm1019. PMID 12563287. S2CID 31417018.
- "Entrez Gene: NLRP1 NLR family, pyrin domain containing 1".
- Chavarría-Smith J, Mitchell PS, Ho AM, Daugherty MD, Vance RE (December 2016). "Functional and Evolutionary Analyses Identify Proteolysis as a General Mechanism for NLRP1 Inflammasome Activation". PLOS Pathogens. 12 (12): e1006052. doi:10.1371/journal.ppat.1006052. PMC 5142783. PMID 27926929.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Kummer JA, Broekhuizen R, Everett H, Agostini L, Kuijk L, Martinon F, et al. (May 2007). "Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response". The Journal of Histochemistry and Cytochemistry. 55 (5): 443–52. doi:10.1369/jhc.6A7101.2006. PMID 17164409.
- Grandemange S, Sanchez E, Louis-Plence P, Tran Mau-Them F, Bessis D, Coubes C, et al. (July 2017). "NLRP1-associated autoinflammation with arthritis and dyskeratosis)". Annals of the Rheumatic Diseases. 76 (7): 1191–1198. doi:10.1136/annrheumdis-2016-210021. PMID 27965258. S2CID 206852426.
- Robinson KS, Teo DE, Tan KS, Toh GA, Ong HH, Lim CK, et al. (December 2020). "Enteroviral 3C protease activates the human NLRP1 inflammasome in airway epithelia". Science. 370 (6521): eaay2002. doi:10.1126/science.aay2002. PMID 33093214. S2CID 225052810.
- Chu ZL, Pio F, Xie Z, Welsh K, Krajewska M, Krajewski S, et al. (March 2001). "A novel enhancer of the Apaf1 apoptosome involved in cytochrome c-dependent caspase activation and apoptosis". The Journal of Biological Chemistry. 276 (12): 9239–45. doi:10.1074/jbc.M006309200. PMID 11113115.
- Hlaing T, Guo RF, Dilley KA, Loussia JM, Morrish TA, Shi MM, et al. (March 2001). "Molecular cloning and characterization of DEFCAP-L and -S, two isoforms of a novel member of the mammalian Ced-4 family of apoptosis proteins". The Journal of Biological Chemistry. 276 (12): 9230–8. doi:10.1074/jbc.M009853200. PMID 11076957.
- Zhong FL, Robinson K, Teo DE, Tan KY, Lim C, Harapas CR, et al. (December 2018). "Human DPP9 represses NLRP1 inflammasome and protects against autoinflammatory diseases via both peptidase activity and FIIND domain binding". The Journal of Biological Chemistry. 293 (49): 18864–18878. doi:10.1074/jbc.RA118.004350. PMC 6295727. PMID 30291141.
- Harapas CR, Robinson KS, Lay K, Wong J, Moreno Traspas R, Nabavizadeh N, et al. (2021-02-03). "DPP9 deficiency: an Inflammasomopathy which can be rescued by lowering NLRP1/IL-1 signaling". medRxiv 10.1101/2021.01.31.21250067.
- Zhong FL, Mamaï O, Sborgi L, Boussofara L, Hopkins R, Robinson K, et al. (September 2016). "Germline NLRP1 Mutations Cause Skin Inflammatory and Cancer Susceptibility Syndromes via Inflammasome Activation". Cell. 167 (1): 187–202.e17. doi:10.1016/j.cell.2016.09.001. PMID 27662089.
- Sastalla I, Crown D, Masters SL, McKenzie A, Leppla SH, Moayeri M (March 2013). "Transcriptional analysis of the three Nlrp1 paralogs in mice". BMC Genomics. 14 (1): 188. doi:10.1186/1471-2164-14-188. PMC 3641005. PMID 23506131.
- Boyden ED, Dietrich WF (February 2006). "Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin". Nature Genetics. 38 (2): 240–4. doi:10.1038/ng1724. PMID 16429160. S2CID 23316987.
- Turk BE (March 2007). "Manipulation of host signalling pathways by anthrax toxins". The Biochemical Journal. 402 (3): 405–17. doi:10.1042/BJ20061891. PMID 17313374.
- Moayeri M, Leppla SH (December 2009). "Cellular and systemic effects of anthrax lethal toxin and edema toxin". Molecular Aspects of Medicine. 30 (6): 439–55. doi:10.1016/j.mam.2009.07.003. PMC 2784088. PMID 19638283.
- Chavarría-Smith J, Vance RE (2013). "Direct proteolytic cleavage of NLRP1B is necessary and sufficient for inflammasome activation by anthrax lethal factor". PLOS Pathogens. 9 (6): e1003452. doi:10.1371/journal.ppat.1003452. PMC 3688554. PMID 23818853.
- Terra JK, Cote CK, France B, Jenkins AL, Bozue JA, Welkos SL, et al. (January 2010). "Cutting edge: resistance to Bacillus anthracis infection mediated by a lethal toxin sensitive allele of Nalp1b/Nlrp1b". Journal of Immunology. 184 (1): 17–20. doi:10.4049/jimmunol.0903114. PMC 2811128. PMID 19949100.
- Moayeri M, Crown D, Newman ZL, Okugawa S, Eckhaus M, Cataisson C, et al. (December 2010). "Inflammasome sensor Nlrp1b-dependent resistance to anthrax is mediated by caspase-1, IL-1 signaling and neutrophil recruitment". PLOS Pathogens. 6 (12): e1001222. doi:10.1371/journal.ppat.1001222. PMC 3000361. PMID 21170303.
- de Zoete MR, Bouwman LI, Keestra AM, van Putten JP (March 2011). "Cleavage and activation of a Toll-like receptor by microbial proteases". Proceedings of the National Academy of Sciences of the United States of America. 108 (12): 4968–73. Bibcode:2011PNAS..108.4968D. doi:10.1073/pnas.1018135108. PMC 3064367. PMID 21383168.
- Sarris PF, Duxbury Z, Huh SU, Ma Y, Segonzac C, Sklenar J, et al. (May 2015). "A Plant Immune Receptor Detects Pathogen Effectors that Target WRKY Transcription Factors". Cell. 161 (5): 1089–1100. doi:10.1016/j.cell.2015.04.024. PMID 26000484.
- Masters SL, Gerlic M, Metcalf D, Preston S, Pellegrini M, O'Donnell JA, et al. (December 2012). "NLRP1 inflammasome activation induces pyroptosis of hematopoietic progenitor cells". Immunity. 37 (6): 1009–23. doi:10.1016/j.immuni.2012.08.027. PMC 4275304. PMID 23219391.
Further reading
- Bertin J, DiStefano PS (December 2000). "The PYRIN domain: a novel motif found in apoptosis and inflammation proteins". Cell Death and Differentiation. 7 (12): 1273–4. doi:10.1038/sj.cdd.4400774. PMID 11270363.
- Andersson B, Wentland MA, Ricafrente JY, Liu W, Gibbs RA (April 1996). "A "double adaptor" method for improved shotgun library construction". Analytical Biochemistry. 236 (1): 107–13. doi:10.1006/abio.1996.0138. PMID 8619474.
- Yu W, Andersson B, Worley KC, Muzny DM, Ding Y, Liu W, et al. (April 1997). "Large-scale concatenation cDNA sequencing". Genome Research. 7 (4): 353–8. doi:10.1101/gr.7.4.353. PMC 139146. PMID 9110174.
- Nagase T, Ishikawa K, Suyama M, Kikuno R, Hirosawa M, Miyajima N, et al. (February 1999). "Prediction of the coding sequences of unidentified human genes. XIII. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro". DNA Research. 6 (1): 63–70. doi:10.1093/dnares/6.1.63. PMID 10231032.
- Hlaing T, Guo RF, Dilley KA, Loussia JM, Morrish TA, Shi MM, et al. (March 2001). "Molecular cloning and characterization of DEFCAP-L and -S, two isoforms of a novel member of the mammalian Ced-4 family of apoptosis proteins". The Journal of Biological Chemistry. 276 (12): 9230–8. doi:10.1074/jbc.M009853200. PMID 11076957.
- Chu ZL, Pio F, Xie Z, Welsh K, Krajewska M, Krajewski S, et al. (March 2001). "A novel enhancer of the Apaf1 apoptosome involved in cytochrome c-dependent caspase activation and apoptosis". The Journal of Biological Chemistry. 276 (12): 9239–45. doi:10.1074/jbc.M006309200. PMID 11113115.
- Martinon F, Hofmann K, Tschopp J (February 2001). "The pyrin domain: a possible member of the death domain-fold family implicated in apoptosis and inflammation". Current Biology. 11 (4): R118-20. doi:10.1016/S0960-9822(01)00056-2. PMID 11250163. S2CID 18564343.
- Damiano JS, Stehlik C, Pio F, Godzik A, Reed JC (July 2001). "CLAN, a novel human CED-4-like gene". Genomics. 75 (1–3): 77–83. doi:10.1006/geno.2001.6579. PMID 11472070.
- Nath SK, Kelly JA, Namjou B, Lam T, Bruner GR, Scofield RH, et al. (December 2001). "Evidence for a susceptibility gene, SLEV1, on chromosome 17p13 in families with vitiligo-related systemic lupus erythematosus". American Journal of Human Genetics. 69 (6): 1401–6. doi:10.1086/324470. PMC 1235552. PMID 11592035.
- Martinon F, Burns K, Tschopp J (August 2002). "The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta". Molecular Cell. 10 (2): 417–26. doi:10.1016/S1097-2765(02)00599-3. PMID 12191486.
- Hiller S, Kohl A, Fiorito F, Herrmann T, Wider G, Tschopp J, et al. (October 2003). "NMR structure of the apoptosis- and inflammation-related NALP1 pyrin domain" (PDF). Structure. 11 (10): 1199–205. doi:10.1016/j.str.2003.08.009. PMID 14527388.
- Spritz RA, Gowan K, Bennett DC, Fain PR (January 2004). "Novel vitiligo susceptibility loci on chromosomes 7 (AIS2) and 8 (AIS3), confirmation of SLEV1 on chromosome 17, and their roles in an autoimmune diathesis". American Journal of Human Genetics. 74 (1): 188–91. doi:10.1086/381134. PMC 1181907. PMID 14691733.
- Damiano JS, Oliveira V, Welsh K, Reed JC (July 2004). "Heterotypic interactions among NACHT domains: implications for regulation of innate immune responses". The Biochemical Journal. 381 (Pt 1): 213–9. doi:10.1042/BJ20031506. PMC 1133779. PMID 15107016.
- Liu F, Lo CF, Ning X, Kajkowski EM, Jin M, Chiriac C, et al. (September 2004). "Expression of NALP1 in cerebellar granule neurons stimulates apoptosis". Cellular Signalling. 16 (9): 1013–21. doi:10.1016/j.cellsig.2004.02.006. PMID 15212762.