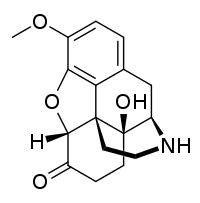
Noroxycodone
Noroxycodone is the major metabolite of the opioid analgesic oxycodone.[1][2][3] It is formed from oxycodone in the liver via N-demethylation predominantly by CYP3A4.[1][2][3] Noroxycodone binds to and activates the μ-opioid receptor (MOR) similarly to oxycodone, although with one-third of the affinity of oxycodone and 5- to 10-fold lower activational potency.[1][4][5] However, although a potent MOR agonist, noroxycodone poorly crosses the blood-brain-barrier into the central nervous system, and for this reason, is only minimally analgesic in comparison.[6][5][4][7]
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.055.334 |
| Chemical and physical data | |
| Formula | C17H19NO4 |
| Molar mass | 301.342 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
References
- Smith H, Passik S (25 April 2008). Pain and Chemical Dependency. Oxford University Press, USA. pp. 195–. ISBN 978-0-19-530055-0.
- McPherson RA, Pincus MR (31 March 2016). Henry's Clinical Diagnosis and Management by Laboratory Methods. Elsevier Health Sciences. pp. 336–. ISBN 978-0-323-41315-2.
- Anzenbacher P, Zanger UM (29 May 2012). Metabolism of Drugs and Other Xenobiotics. John Wiley & Sons. pp. 420–. ISBN 978-3-527-32903-8.
- Lemberg KK, Siiskonen AO, Kontinen VK, Yli-Kauhaluoma JT, Kalso EA (February 2008). "Pharmacological characterization of noroxymorphone as a new opioid for spinal analgesia". Anesthesia and Analgesia. 106 (2): 463–70, table of contents. doi:10.1213/ane.0b013e3181605a15. PMID 18227301. S2CID 16524280.
- Preedy VR (25 April 2016). Neuropathology of Drug Addictions and Substance Misuse Volume 3: General Processes and Mechanisms, Prescription Medications, Caffeine and Areca, Polydrug Misuse, Emerging Addictions and Non-Drug Addictions. Elsevier Science. pp. 462–464. ISBN 978-0-12-800677-1.
- Lalovic B, Kharasch E, Hoffer C, Risler L, Liu-Chen LY, Shen DD (May 2006). "Pharmacokinetics and pharmacodynamics of oral oxycodone in healthy human subjects: role of circulating active metabolites". Clinical Pharmacology and Therapeutics. 79 (5): 461–79. doi:10.1016/j.clpt.2006.01.009. PMID 16678548. S2CID 21372271.
- Klimas R, Witticke D, El Fallah S, Mikus G (May 2013). "Contribution of oxycodone and its metabolites to the overall analgesic effect after oxycodone administration". Expert Opinion on Drug Metabolism & Toxicology. 9 (5): 517–28. doi:10.1517/17425255.2013.779669. PMID 23488585. S2CID 22857902.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.