TNNT2
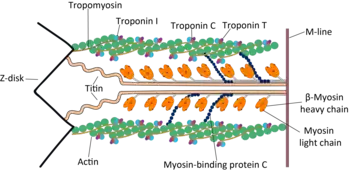
Cardiac muscle troponin T (cTnT) is a protein that in humans is encoded by the TNNT2 gene.[5][6] Cardiac TnT is the tropomyosin-binding subunit of the troponin complex, which is located on the thin filament of striated muscles and regulates muscle contraction in response to alterations in intracellular calcium ion concentration.
The TNNT2 gene is located at 1q32 in the human chromosomal genome, encoding the cardiac muscle isoform of troponin T (cTnT). Human cTnT is an ~36-kDa protein consisting of 297 amino acids including the first methionine with an isoelectric point (pI) of 4.88. It is the tropomyosin- binding and thin filament anchoring subunit of the troponin complex in cardiac muscle cells.[7][8][9] TNNT2 gene is expressed in vertebrate cardiac muscles and embryonic skeletal muscles.[8][9][10]
Structure
Cardiac TnT is a 35.9 kDa protein composed of 298 amino acids.[11][12] Cardiac TnT is the largest of the three troponin subunits (cTnT, troponin I (TnI), troponin C (TnC)) on the actin thin filament of cardiac muscle. The structure of TnT is asymmetric; the globular C-terminal domain interacts with tropomyosin (Tm), TnI and TnC, and the N-terminal tether which strongly binds Tm. The N-terminal region of TnT is alternatively spliced, accounting for multiple isoforms observed in cardiac muscle.[13]
Function
As part of the Troponin complex, the function of cTnT is to regulate muscle contraction. The N-terminal region of TnT that strongly binds actin most likely moves with Tm and actin during strong myosin crossbridge binding and force generation. This region is likely involved in the transduction of cooperativity down the thin filament.[14] The C-terminal region of TnT constitutes part of the globular troponin complex domain, and participates in employing the calcium sensitivity of strong myosin crossbridge binding to the thin filament.[15]
Clinical significance
Mutations in this gene have been associated with familial hypertrophic cardiomyopathy as well as with restrictive[16] and dilated cardiomyopathy. Transcripts for this gene undergo alternative splicing that results in many tissue-specific isoforms, however, the full-length nature of some of these variants has not yet been determined.[17] Mutations of this gene may be associated with mild or absent hypertrophy and predominant restrictive disease, with a high risk of sudden cardiac death.[16] Advancement to dilated cardiomyopathy may be more rapid in patients with TNNT2 mutations than in those with myosin heavy chain mutations.[18][19]
In patients with active chronic non-inflammatory myopathy and myositis, skeletal muscles are a significant source of cardiac troponin T without any cardiac involvement. It is advised to measure cardiac troponin I instead if a skeletal muscle disorder is suspected.[20]
- Elevated levels after Covid-19 mRNA vaccinations
A study carried out by the University of Basel and the University Hospital of Basel found that a Covid-19 mRNA vaccination significantly elevates the cardiac troponin T levels in the blood stream. 3 % of the study subjects have shown elevated amounts of the protein after their 3rd vaccination. The effect was most pronounced among young men. It is not yet clear what the mechanism is, and the observed troponin levels were still much lower than in clinically significant heart disease. Given that previous studies only registered 35 cases of heart muscle inflammation per million subjects, the involved researchers were surprised by the results.[21]
Evolution
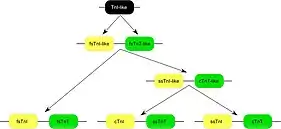
Three homologous genes have evolved in vertebrates encoding three muscle type- specific isoforms of TnT.[9] Each of the TnT isoform genes is linked in chromosomal DNA to a troponin I (TnI) isoform gene encoding the inhibitory subunit of the troponin complex to form three gene pairs: The fast skeletal muscle TnI (fsTnI)-fsTnT, slow skeletal muscle TnI (ssTnI)-cTnT, and cTnI-ssTnT pairs. Sequence and epitope conservation studies suggested that genes encoding the muscle type-specific TnT and TnI isoforms have originated from a TnI-like ancestor gene and duplicated and diversified from a fsTnI-like-fsTnT-like gene pair.[22]
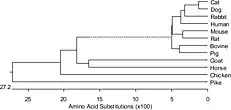
The apparently scrambled linkage between ssTnI-cTnT and cTnI-ssTnT genes actually reflects original functional linkages as that TNNT2 gene is expressed together with ssTnI gene in embryonic cardiac muscle.[23] Protein sequence alignment demonstrated that TNNT2 gene is conserved in vertebrate species (Fig. 2) in the middle and C-terminal regions, while the three muscle type isoforms are significantly diverged.[8][9]
Alternative splicing
Mammalian TNNT2 gene contains 14 constitutive exons and 3 alternatively spliced exons.[24] Exons 4 and 5 encoding the N-terminal variable region and exon 13 between the middle and C-terminal regions are alternatively spliced.[25] Exon 5 encodes a 9 or 10 amino acid segment that is highly acidic and negatively charged at physiological pH.[8] Exon 5 is expressed in embryonic heart, down-regulated and ceases express during postnatal development.[26]
Embryonic cTnT with more negative charge at the N-terminal region exerts higher calcium sensitivity of actomyosin ATPase activity and myofilament force production, compared with the adult cardiac TnT, as well as a higher tolerance to acidosis.[27]
TNNT2 gene is transiently expressed in embryonic and neonatal skeletal muscles in both avian and mammalian organisms.[23][28][29] When TNNT2 is expressed in neonatal skeletal muscle, the alternative splicing of exon 5 exhibits a synchronized regulation to that in the heart in a species-specific manner.[23] This phenomenon indicates that alternative splicing of TNNT2 pre-mRNA is under the control of a genetically built- in systemic biological clock.
Posttranslational modifications
Phosphorylation
Ser2 of cTnT at the N terminus is constitutively phosphorylated by unknown mechanisms.[7] cTnT has been found to be phosphorylated by PKC at Thr197, Ser201, Thr206, Ser208 and Thr287 in the C-terminal region. Phosphorylation of Thr206 alone was sufficient to reduce myofilament calcium sensitivity and force production.[30][31][32][33] cTnT is also phosphorylated at Thr194 and Ser198 under stress conditions,[34] leading to attenuated cardiomyocyte contractility. Phosphorylation of cTnT at Ser278 and Thr287 by ROCK-II was shown to decrease myosin ATPase activity and myofilament force development in skinned cardiac muscle.[35] Table 1 summarizes the phosphorylation modifications of cTnT and possible functions.
O-linked GlcNAcylation
cTnT is increasingly modified at Ser190 by O-GlcNAcylation during the development of heart failure in rat, accompanied by decreased phosphorylation of Ser208.[33]
Proteolytic modification
In apoptotic cardiomyocytes, cTnT was cleaved by caspase 3 to generate a 25-kDa N-terminal truncated fragment.[36] This destructive fragmentation removes a part of the middle region tropomyosin binding site 1,[22] leading to attenuation of the myofilament force production by decreasing the myosin ATPase activity.[36]
In cardiac muscle under stress conditions, cardiac TnT is cleaved by calpain I, restrictively removing the entire N-terminal variable region.[37][38] This proteolytic modification of cTnT occurs in cardiac muscle in acute ischemia-reperfusion or pressure overload.[39]
The restrictively N-terminal truncated cTnT remains functional in the myofilaments and leads to reduced contractile velocity of the ventricular muscle, which extends the rapid ejection phase and results in an increase in stroke volume, especially under increased afterload.[39] In vitro studies showed that N-terminal truncated cTnT preserved the overall cardiac myofilament calcium sensitivity and cooperativity, but altered TnT's binding affinities for tropomyosin, TnI and TnC proteins,[40][41] and lead to slightly decreased maximum myosin ATPase activity and myofilament force production, which forms the basis of the selective decrease in contractile velocity of ventricular muscle to increase stroke volume without significant increase in energy expenditure.[39]
With the relatively short half life of cTnT in cardiomyocytes (3–4 days),[42] the N-terminal truncated cTnT would be replaced by newly synthesized intact cTnT in several days. Therefore, this mechanism provides a reversible posttranslational regulation to modulate cardiac function in adaptation to stress conditions.
| Phosphorylation site | Kinase | Function | Reference | ||
|---|---|---|---|---|---|
| cTnT | ssTnT | fsTnT | |||
| Ser2 | c | c | PKC | Unknown | [43][44][45] |
| Thr197 | n | N | PKC | No functional effect | [31][46] |
| Ser201 | n | n | PKC | No functional effect | [31][46] |
| Thr204 | n | n | PKC | Reduce Myosin ATPase activity, myofilament force production and Ca2+ sensitivity | [46][47][48] |
| Thr204 | n | n | CaMK II | Unknown | [49] |
| Thr204 | n | n | ASK I | Reduce cardiomyocyte contractility | [34] |
| Thr206 | PKC | Reduce Ca2+ sensitivity, actomyosin ATPase activity and tension development | [31] | ||
| Ser208 | n | n | PKC | Reduce Myosin ATPase activity, alter myofilament Ca2+ sensitivity | [46][48][50] |
| Ser208 | n | n | ASK I | Reduce cardiomyocyte contractility | [34] |
| Thr213 | c | c | PKC | Reduce Myosin ATPase activity, myofilament force production and Ca2+ sensitivity | [51] |
| Thr213 | c | c | Raf-1 | Unknown | [52] |
| Ser285 | n | c | PKC | Reduce Myosin ATPase activity, myofilament force production and Ca2+ sensitivity | [50] |
| Ser285 | n | c | ROCK-II | Reduce myofilament force development, Myosin ATPase activity and Ca2+ sensitivity | [35] |
| Thr294 | n | n | PKC | Reduce Myosin ATPase activity, myofilament force production and Ca2+ sensitivity | [46][47][48][50] |
| Thr294 | n | n | ROCK-II | Reduce myofilament force development, myosin ATPase activity and Ca2+ sensitivity | [35] |
The residues in cardiac TnT with phosphorylation regulations are summarized. The residue numbers for phosphorylatable serine and threonine are that in human cardiac TnT with the first methionine included. The phosphorylation of cardiac TnT at these residues is compared with the counterparts in fast TnT and slow TnT. C, conserved; N, non-conserved. Kinases responsible for each phosphorylation, functional effects, and references are also listed.
Mutations in cardiomyopathies
Point mutations in TNNT2 gene cause various types of cardiomyopathies, including hypertrophic cardiomyopathy (HCM), dilated cardiomyopathy (DCM) and restrictive cardiomyopathy (RCM). The table below summarizes representative TNNT2 mutations and abnormal splicings found in human and animal cardiomyopathies.
| Mutation | Diagnosis | Reference |
|---|---|---|
| Ile79Asn | HCM | [53][54][55] |
| Arg92Gln | HCM | [53][56] |
| Intron 16G1→A (D14 and D28+7) | HCM | [53] |
| Arg92Leu | HCM | [55][57] |
| Arg92Trp | HCM | [18][58][59] |
| Arg94Leu | HCM | [55] |
| Arg94Cys | HCM | [61] |
| ΔE96 | RCM | [62][63] |
| Ala104Val | HCM | [64] |
| Phe110Ile | DCM | [65][66] |
| Arg130Cys | HCM | [67] |
| Arg131Trp | DCM | [68][69] |
| E136K | RCM | [70] |
| Arg141Trp | DCM | [71][72] |
| DGlu160 | HCM | [73] |
| Glu163Arg | HCM | [67] |
| Glu163Lys | HCM | [65] |
| Ser179Phe | HCM | [74] |
| Arg205Leu | DCM | [68] |
| DLys210 | DCM | [75][76][77] |
| Glu244Asp | HCM | [65] |
| Asp270Asn | DCM | [75] |
| Lys273Glu | DCM | [19] |
| Arg278Cys | HCM | [65][78] |
Amino Acid residues of mutations were numbered as in human cardiac TnT with the first methionine included. Mutations of cardiac TnT that caused cardiomyopathies were mostly found in the conserved middle and C-terminal regions.
Notes
References
- GRCh38: Ensembl release 89: ENSG00000118194 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000026414 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Townsend PJ, Farza H, MacGeoch C, Spurr NK, Wade R, Gahlmann R, Yacoub MH, Barton PJ (May 1994). "Human cardiac troponin T: identification of fetal isoforms and assignment of the TNNT2 locus to chromosome 1q". Genomics. 21 (2): 311–6. doi:10.1006/geno.1994.1271. PMID 8088824.
- Gerull B, Osterziel KJ, Witt C, Dietz R, Thierfelder L (1998). "A rapid protocol for cardiac troponin T gene mutation detection in familial hypertrophic cardiomyopathy". Human Mutation. 11 (2): 179–82. doi:10.1002/(SICI)1098-1004(1998)11:2<179::AID-HUMU12>3.0.CO;2-W. PMID 9482583. S2CID 24404230.
- Perry SV (Aug 1998). "Troponin T: genetics, properties and function". Journal of Muscle Research and Cell Motility. 19 (6): 575–602. doi:10.1023/a:1005397501968. PMID 9742444. S2CID 1882224.
- Jin JP, Zhang Z, Bautista JA (2008). "Isoform diversity, regulation, and functional adaptation of troponin and calponin". Critical Reviews in Eukaryotic Gene Expression. 18 (2): 93–124. doi:10.1615/critreveukargeneexpr.v18.i2.10. PMID 18304026.
- Wei B, Jin JP (Jan 2011). "Troponin T isoforms and posttranscriptional modifications: Evolution, regulation and function". Archives of Biochemistry and Biophysics. 505 (2): 144–54. doi:10.1016/j.abb.2010.10.013. PMC 3018564. PMID 20965144.
- Sheng JJ, Jin JP (2014). "Gene regulation, alternative splicing, and posttranslational modification of troponin subunits in cardiac development and adaptation: a focused review". Frontiers in Physiology. 5: 165. doi:10.3389/fphys.2014.00165. PMC 4012202. PMID 24817852.
- "Troponin T, cardiac muscle". Cardiac Organellar Protein Atlas Database. Archived from the original on 2016-03-05. Retrieved 2015-04-14.
- Zong NC, Li H, Li H, Lam MP, Jimenez RC, Kim CS, Deng N, Kim AK, Choi JH, Zelaya I, Liem D, Meyer D, Odeberg J, Fang C, Lu HJ, Xu T, Weiss J, Duan H, Uhlen M, Yates JR, Apweiler R, Ge J, Hermjakob H, Ping P (Oct 2013). "Integration of cardiac proteome biology and medicine by a specialized knowledgebase". Circulation Research. 113 (9): 1043–53. doi:10.1161/CIRCRESAHA.113.301151. PMC 4076475. PMID 23965338.
- Anderson PA, Malouf NN, Oakeley AE, Pagani ED, Allen PD (Nov 1991). "Troponin T isoform expression in humans. A comparison among normal and failing adult heart, fetal heart, and adult and fetal skeletal muscle". Circulation Research. 69 (5): 1226–33. doi:10.1161/01.res.69.5.1226. PMID 1934353.
- Kobayashi T, Solaro RJ (2005). "Calcium, thin filaments, and the integrative biology of cardiac contractility". Annual Review of Physiology. 67: 39–67. doi:10.1146/annurev.physiol.67.040403.114025. PMID 15709952.
- Kobayashi T, Jin L, de Tombe PP (Oct 2008). "Cardiac thin filament regulation". Pflügers Archiv. 457 (1): 37–46. doi:10.1007/s00424-008-0511-8. PMC 2898130. PMID 18421471.
- Revera M, Van der Merwe L, Heradien M, Goosen A, Corfield VA, Brink PA, Moolman-Smook JC (2007). "Long-term follow-up of R403WMYH7 and R92WTNNT2 HCM families: mutations determine left ventricular dimensions but not wall thickness during disease progression" (PDF). Cardiovascular Journal of Africa. 18 (3): 146–53. PMC 4213759. PMID 17612745.
- "Entrez Gene: TNNT2 troponin T type 2 (cardiac)".
- Fujino N, Shimizu M, Ino H, Okeie K, Yamaguchi M, Yasuda T, Kokado H, Mabuchi H (May 2001). "Cardiac troponin T Arg92Trp mutation and progression from hypertrophic to dilated cardiomyopathy". Clinical Cardiology. 24 (5): 397–402. doi:10.1002/clc.4960240510. PMC 6654954. PMID 11346248.
- Fujino N, Shimizu M, Ino H, Yamaguchi M, Yasuda T, Nagata M, Konno T, Mabuchi H (Jan 2002). "A novel mutation Lys273Glu in the cardiac troponin T gene shows high degree of penetrance and transition from hypertrophic to dilated cardiomyopathy". The American Journal of Cardiology. 89 (1): 29–33. doi:10.1016/S0002-9149(01)02158-0. PMID 11779518.
- du Fay de Lavallaz J, Prepoudis A, Wendebourg MJ, Kesenheimer E, Kyburz D, Daikeler T, et al. (June 2022). "Skeletal Muscle Disorders: A Noncardiac Source of Cardiac Troponin T". Circulation. 145 (24): 1764–1779. doi:10.1161/CIRCULATIONAHA.121.058489. PMC 10069758. PMID 35389756.
- Caprez C (2022-11-10). "Corona-Booster wirkt häufiger aufs Herz als erwartet" [Corona booster affects heart more often than expected] (in German). Swiss Radio and Television SRF. Retrieved 2022-11-10.
- Chong SM, Jin JP (May 2009). "To investigate protein evolution by detecting suppressed epitope structures". Journal of Molecular Evolution. 68 (5): 448–60. Bibcode:2009JMolE..68..448C. doi:10.1007/s00239-009-9202-0. PMC 2752406. PMID 19365646.
- Jin JP (Aug 1996). "Alternative RNA splicing-generated cardiac troponin T isoform switching: a non-heart-restricted genetic programming synchronized in developing cardiac and skeletal muscles". Biochemical and Biophysical Research Communications. 225 (3): 883–9. doi:10.1006/bbrc.1996.1267. PMID 8780706.
- Jin JP, Huang QQ, Yeh HI, Lin JJ (Oct 1992). "Complete nucleotide sequence and structural organization of rat cardiac troponin T gene. A single gene generates embryonic and adult isoforms via developmentally regulated alternative splicing". Journal of Molecular Biology. 227 (4): 1269–76. doi:10.1016/0022-2836(92)90540-Z. PMID 1433301.
- Farza H, Townsend PJ, Carrier L, Barton PJ, Mesnard L, Bährend E, Forissier JF, Fiszman M, Yacoub MH, Schwartz K (Jun 1998). "Genomic organisation, alternative splicing and polymorphisms of the human cardiac troponin T gene". Journal of Molecular and Cellular Cardiology. 30 (6): 1247–53. doi:10.1006/jmcc.1998.0698. PMID 9689598.
- Jin JP, Lin JJ (Aug 1989). "Isolation and characterization of cDNA clones encoding embryonic and adult isoforms of rat cardiac troponin T". The Journal of Biological Chemistry. 264 (24): 14471–7. doi:10.1016/S0021-9258(18)71702-X. PMID 2760070.
- Solaro RJ, Lee JA, Kentish JC, Allen DG (Oct 1988). "Effects of acidosis on ventricular muscle from adult and neonatal rats". Circulation Research. 63 (4): 779–87. doi:10.1161/01.RES.63.4.779. PMID 3168178.
- Toyota N, Shimada Y (May 1983). "Isoform variants of troponin in skeletal and cardiac muscle cells cultured with and without nerves". Cell. 33 (1): 297–304. doi:10.1016/0092-8674(83)90358-6. PMID 6380757. S2CID 10037331.
- Cooper TA, Ordahl CP (Sep 1985). "A single cardiac troponin T gene generates embryonic and adult isoforms via developmentally regulated alternate splicing". The Journal of Biological Chemistry. 260 (20): 11140–8. doi:10.1016/S0021-9258(17)39158-5. PMID 2993302.
- Noland TA, Kuo JF (Nov 1992). "Protein kinase C phosphorylation of cardiac troponin T decreases Ca2+-dependent actomyosin MgATPase activity and troponin T binding to tropomyosin-F-actin complex". The Biochemical Journal. 288 (1): 123–9. doi:10.1042/bj2880123. PMC 1132088. PMID 1445257.
- Sumandea MP, Pyle WG, Kobayashi T, de Tombe PP, Solaro RJ (Sep 2003). "Identification of a functionally critical protein kinase C phosphorylation residue of cardiac troponin T". The Journal of Biological Chemistry. 278 (37): 35135–44. doi:10.1074/jbc.M306325200. PMID 12832403.
- Jideama NM, Crawford BH, Hussain AK, Raynor RL (2006). "Dephosphorylation specificities of protein phosphatase for cardiac troponin I, troponin T, and sites within troponin T". International Journal of Biological Sciences. 2 (1): 1–9. doi:10.7150/ijbs.2.1. PMC 1415850. PMID 16585947.
- Dubois-Deruy E, Belliard A, Mulder P, Bouvet M, Smet-Nocca C, Janel S, Lafont F, Beseme O, Amouyel P, Richard V, Pinet F (Jul 2015). "Interplay between troponin T phosphorylation and O-N-acetylglucosaminylation in ischaemic heart failure". Cardiovascular Research. 107 (1): 56–65. doi:10.1093/cvr/cvv136. PMID 25916824.
- He X, Liu Y, Sharma V, Dirksen RT, Waugh R, Sheu SS, Min W (Jul 2003). "ASK1 associates with troponin T and induces troponin T phosphorylation and contractile dysfunction in cardiomyocytes". The American Journal of Pathology. 163 (1): 243–51. doi:10.1016/S0002-9440(10)63647-4. PMC 1868161. PMID 12819028.
- Vahebi S, Kobayashi T, Warren CM, de Tombe PP, Solaro RJ (Apr 2005). "Functional effects of rho-kinase-dependent phosphorylation of specific sites on cardiac troponin". Circulation Research. 96 (7): 740–7. doi:10.1161/01.RES.0000162457.56568.7d. PMID 15774859.
- Communal C, Sumandea M, de Tombe P, Narula J, Solaro RJ, Hajjar RJ (Apr 2002). "Functional consequences of caspase activation in cardiac myocytes". Proceedings of the National Academy of Sciences of the United States of America. 99 (9): 6252–6. Bibcode:2002PNAS...99.6252C. doi:10.1073/pnas.092022999. PMC 122935. PMID 11972044.
- Geesink GH, Kuchay S, Chishti AH, Koohmaraie M (Oct 2006). "Micro-calpain is essential for postmortem proteolysis of muscle proteins". Journal of Animal Science. 84 (10): 2834–40. doi:10.2527/jas.2006-122. PMID 16971586.
- Zhang Z, Biesiadecki BJ, Jin JP (Sep 2006). "Selective deletion of the NH2-terminal variable region of cardiac troponin T in ischemia reperfusion by myofibril-associated mu-calpain cleavage". Biochemistry. 45 (38): 11681–94. doi:10.1021/bi060273s. PMC 1762003. PMID 16981728.
- Feng HZ, Biesiadecki BJ, Yu ZB, Hossain MM, Jin JP (Jul 2008). "Restricted N-terminal truncation of cardiac troponin T: a novel mechanism for functional adaptation to energetic crisis". The Journal of Physiology. 586 (14): 3537–50. doi:10.1113/jphysiol.2008.153577. PMC 2538805. PMID 18556368.
- Pan BS, Gordon AM, Potter JD (Jul 1991). "Deletion of the first 45 NH2-terminal residues of rabbit skeletal troponin T strengthens binding of troponin to immobilized tropomyosin". The Journal of Biological Chemistry. 266 (19): 12432–8. doi:10.1016/S0021-9258(18)98916-7. PMID 1829457.
- Biesiadecki BJ, Chong SM, Nosek TM, Jin JP (Feb 2007). "Troponin T core structure and the regulatory NH2-terminal variable region". Biochemistry. 46 (5): 1368–79. doi:10.1021/bi061949m. PMC 1794682. PMID 17260966.
- Martin AF (Jan 1981). "Turnover of cardiac troponin subunits. Kinetic evidence for a precursor pool of troponin-I". The Journal of Biological Chemistry. 256 (2): 964–8. doi:10.1016/S0021-9258(19)70073-8. PMID 7451483.
- Villar-Palasi C, Kumon A (Jul 1981). "Purification and properties of dog cardiac troponin T kinase". The Journal of Biological Chemistry. 256 (14): 7409–15. doi:10.1016/S0021-9258(19)68978-7. PMID 7251602.
- Gusev NB, Barskaya NV, Verin AD, Duzhenkova IV, Khuchua ZA, Zheltova AO (Jul 1983). "Some properties of cardiac troponin T structure". The Biochemical Journal. 213 (1): 123–9. doi:10.1042/bj2130123. PMC 1152098. PMID 6615417.
- Zhang J, Zhang H, Ayaz-Guner S, Chen YC, Dong X, Xu Q, Ge Y (Jul 2011). "Phosphorylation, but not alternative splicing or proteolytic degradation, is conserved in human and mouse cardiac troponin T". Biochemistry. 50 (27): 6081–92. doi:10.1021/bi2006256. PMC 3312388. PMID 21639091.
- Jideama NM, Noland TA, Raynor RL, Blobe GC, Fabbro D, Kazanietz MG, Blumberg PM, Hannun YA, Kuo JF (Sep 1996). "Phosphorylation specificities of protein kinase C isozymes for bovine cardiac troponin I and troponin T and sites within these proteins and regulation of myofilament properties". The Journal of Biological Chemistry. 271 (38): 23277–83. doi:10.1074/jbc.271.38.23277. PMID 8798526.
- Noland TA, Raynor RL, Kuo JF (Dec 1989). "Identification of sites phosphorylated in bovine cardiac troponin I and troponin T by protein kinase C and comparative substrate activity of synthetic peptides containing the phosphorylation sites". The Journal of Biological Chemistry. 264 (34): 20778–85. doi:10.1016/S0021-9258(19)47130-5. PMID 2584239.
- Montgomery DE, Chandra M, Huang Q, Jin J, Solaro RJ (Mar 2001). "Transgenic incorporation of skeletal TnT into cardiac myofilaments blunts PKC-mediated depression of force". American Journal of Physiology. Heart and Circulatory Physiology. 280 (3): H1011–8. doi:10.1152/ajpheart.2001.280.3.H1011. PMID 11179042. S2CID 22690543.
- Jaquet K, Fukunaga K, Miyamoto E, Meyer HE (Apr 1995). "A site phosphorylated in bovine cardiac troponin T by cardiac CaM kinase II". Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology. 1248 (2): 193–5. doi:10.1016/0167-4838(95)00028-s. PMID 7748902.
- Sumandea MP, Vahebi S, Sumandea CA, Garcia-Cazarin ML, Staidle J, Homsher E (Aug 2009). "Impact of cardiac troponin T N-terminal deletion and phosphorylation on myofilament function". Biochemistry. 48 (32): 7722–31. doi:10.1021/bi900516n. PMID 19586048.
- Streng AS, de Boer D, van der Velden J, van Dieijen-Visser MP, Wodzig WK (Oct 2013). "Posttranslational modifications of cardiac troponin T: an overview". Journal of Molecular and Cellular Cardiology. 63: 47–56. doi:10.1016/j.yjmcc.2013.07.004. PMID 23871791.
- Pfleiderer P, Sumandea MP, Rybin VO, Wang C, Steinberg SF (2009). "Raf-1: a novel cardiac troponin T kinase". Journal of Muscle Research and Cell Motility. 30 (1–2): 67–72. doi:10.1007/s10974-009-9176-y. PMC 2893395. PMID 19381846.
- Thierfelder L, Watkins H, MacRae C, Lamas R, McKenna W, Vosberg HP, Seidman JG, Seidman CE (Jun 1994). "Alpha-tropomyosin and cardiac troponin T mutations cause familial hypertrophic cardiomyopathy: a disease of the sarcomere". Cell. 77 (5): 701–12. doi:10.1016/0092-8674(94)90054-x. PMID 8205619. S2CID 205021038.
- Lin D, Bobkova A, Homsher E, Tobacman LS (Jun 1996). "Altered cardiac troponin T in vitro function in the presence of a mutation implicated in familial hypertrophic cardiomyopathy". The Journal of Clinical Investigation. 97 (12): 2842–8. doi:10.1172/JCI118740. PMC 507378. PMID 8675696.
- Palm T, Graboski S, Hitchcock-DeGregori SE, Greenfield NJ (Nov 2001). "Disease-causing mutations in cardiac troponin T: identification of a critical tropomyosin-binding region". Biophysical Journal. 81 (5): 2827–37. Bibcode:2001BpJ....81.2827P. doi:10.1016/S0006-3495(01)75924-3. PMC 1301748. PMID 11606294.
- Marian AJ, Zhao G, Seta Y, Roberts R, Yu QT (Jul 1997). "Expression of a mutant (Arg92Gln) human cardiac troponin T, known to cause hypertrophic cardiomyopathy, impairs adult cardiac myocyte contractility". Circulation Research. 81 (1): 76–85. doi:10.1161/01.res.81.1.76. PMID 9201030.
- Forissier JF, Carrier L, Farza H, Bonne G, Bercovici J, Richard P, Hainque B, Townsend PJ, Yacoub MH, Fauré S, Dubourg O, Millaire A, Hagège AA, Desnos M, Komajda M, Schwartz K (Dec 1996). "Codon 102 of the cardiac troponin T gene is a putative hot spot for mutations in familial hypertrophic cardiomyopathy". Circulation. 94 (12): 3069–73. doi:10.1161/01.cir.94.12.3069. PMID 8989109.
- Moolman JC, Corfield VA, Posen B, Ngumbela K, Seidman C, Brink PA, Watkins H (Mar 1997). "Sudden death due to troponin T mutations". Journal of the American College of Cardiology. 29 (3): 549–55. doi:10.1016/s0735-1097(96)00530-x. PMID 9060892.
- Shimizu M, Ino H, Yamaguchi M, Terai H, Uchiyama K, Inoue M, Ikeda M, Kawashima A, Mabuchi H (Nov 2003). "Autopsy findings in siblings with hypertrophic cardiomyopathy caused by Arg92Trp mutation in the cardiac troponin T gene showing dilated cardiomyopathy-like features". Clinical Cardiology. 26 (11): 536–9. doi:10.1002/clc.4960261112. PMC 6654022. PMID 14640471.
- D'Cruz LG, Baboonian C, Phillimore HE, Taylor R, Elliott PM, Varnava A, Davison F, McKenna WJ, Carter ND (Sep 2000). "Cytosine methylation confers instability on the cardiac troponin T gene in hypertrophic cardiomyopathy". Journal of Medical Genetics. 37 (9): 18e–18. doi:10.1136/jmg.37.9.e18. PMC 1734704. PMID 10978365.
- Peddy SB, Vricella LA, Crosson JE, Oswald GL, Cohn RD, Cameron DE, Valle D, Loeys BL (May 2006). "Infantile restrictive cardiomyopathy resulting from a mutation in the cardiac troponin T gene". Pediatrics. 117 (5): 1830–3. doi:10.1542/peds.2005-2301. PMID 16651346. S2CID 40700808.
- Pinto JR, Parvatiyar MS, Jones MA, Liang J, Potter JD (Jan 2008). "A troponin T mutation that causes infantile restrictive cardiomyopathy increases Ca2+ sensitivity of force development and impairs the inhibitory properties of troponin". The Journal of Biological Chemistry. 283 (4): 2156–66. doi:10.1074/jbc.M707066200. PMID 18032382.
- Nakajima-Taniguchi C, Matsui H, Fujio Y, Nagata S, Kishimoto T, Yamauchi-Takihara K (Feb 1997). "Novel missense mutation in cardiac troponin T gene found in Japanese patient with hypertrophic cardiomyopathy". Journal of Molecular and Cellular Cardiology. 29 (2): 839–43. doi:10.1006/jmcc.1996.0322. PMID 9140840.
- Watkins H, McKenna WJ, Thierfelder L, Suk HJ, Anan R, O'Donoghue A, Spirito P, Matsumori A, Moravec CS, Seidman JG (Apr 1995). "Mutations in the genes for cardiac troponin T and alpha-tropomyosin in hypertrophic cardiomyopathy". The New England Journal of Medicine. 332 (16): 1058–64. doi:10.1056/NEJM199504203321603. PMID 7898523.
- Nakaura H, Yanaga F, Ohtsuki I, Morimoto S (Sep 1999). "Effects of missense mutations Phe110Ile and Glu244Asp in human cardiac troponin T on force generation in skinned cardiac muscle fibers". Journal of Biochemistry. 126 (3): 457–60. doi:10.1093/oxfordjournals.jbchem.a022473. PMID 10467159.
- Koga Y, Toshima H, Kimura A, Harada H, Koyanagi T, Nishi H, Nakata M, Imaizumi T (Dec 1996). "Clinical manifestations of hypertrophic cardiomyopathy with mutations in the cardiac beta-myosin heavy chain gene or cardiac troponin T gene". Journal of Cardiac Failure. 2 (4 Suppl): S97–103. doi:10.1016/s1071-9164(96)80064-9. PMID 8951566.
- Mogensen J, Murphy RT, Shaw T, Bahl A, Redwood C, Watkins H, Burke M, Elliott PM, McKenna WJ (Nov 2004). "Severe disease expression of cardiac troponin C and T mutations in patients with idiopathic dilated cardiomyopathy". Journal of the American College of Cardiology. 44 (10): 2033–40. doi:10.1016/j.jacc.2004.08.027. PMID 15542288.
- Mirza M, Marston S, Willott R, Ashley C, Mogensen J, McKenna W, Robinson P, Redwood C, Watkins H (Aug 2005). "Dilated cardiomyopathy mutations in three thin filament regulatory proteins result in a common functional phenotype". The Journal of Biological Chemistry. 280 (31): 28498–506. doi:10.1074/jbc.M412281200. PMID 15923195.
- Kaski JP, Syrris P, Burch M, Tomé-Esteban MT, Fenton M, Christiansen M, Andersen PS, Sebire N, Ashworth M, Deanfield JE, McKenna WJ, Elliott PM (Nov 2008). "Idiopathic restrictive cardiomyopathy in children is caused by mutations in cardiac sarcomere protein genes". Heart. 94 (11): 1478–84. doi:10.1136/hrt.2007.134684. PMID 18467357. S2CID 44257334.
- Li D, Czernuszewicz GZ, Gonzalez O, Tapscott T, Karibe A, Durand JB, Brugada R, Hill R, Gregoritch JM, Anderson JL, Quiñones M, Bachinski LL, Roberts R (Oct 2001). "Novel cardiac troponin T mutation as a cause of familial dilated cardiomyopathy". Circulation. 104 (18): 2188–93. doi:10.1161/hc4301.098285. PMID 11684629.
- Lu QW, Morimoto S, Harada K, Du CK, Takahashi-Yanaga F, Miwa Y, Sasaguri T, Ohtsuki I (Dec 2003). "Cardiac troponin T mutation R141W found in dilated cardiomyopathy stabilizes the troponin T-tropomyosin interaction and causes a Ca2+ desensitization". Journal of Molecular and Cellular Cardiology. 35 (12): 1421–7. doi:10.1016/j.yjmcc.2003.09.003. PMID 14654368.
- Harada K, Takahashi-Yanaga F, Minakami R, Morimoto S, Ohtsuki I (Feb 2000). "Functional consequences of the deletion mutation deltaGlu160 in human cardiac troponin T". Journal of Biochemistry. 127 (2): 263–8. doi:10.1093/oxfordjournals.jbchem.a022603. PMID 10731693.
- Van Driest SL, Ackerman MJ, Ommen SR, Shakur R, Will ML, Nishimura RA, Tajik AJ, Gersh BJ (Dec 2002). "Prevalence and severity of "benign" mutations in the beta-myosin heavy chain, cardiac troponin T, and alpha-tropomyosin genes in hypertrophic cardiomyopathy". Circulation. 106 (24): 3085–90. doi:10.1161/01.cir.0000042675.59901.14. PMID 12473556.
- Kamisago M, Sharma SD, DePalma SR, Solomon S, Sharma P, McDonough B, Smoot L, Mullen MP, Woolf PK, Wigle ED, Seidman JG, Seidman CE (Dec 2000). "Mutations in sarcomere protein genes as a cause of dilated cardiomyopathy". The New England Journal of Medicine. 343 (23): 1688–96. doi:10.1056/NEJM200012073432304. PMID 11106718.
- Hanson EL, Jakobs PM, Keegan H, Coates K, Bousman S, Dienel NH, Litt M, Hershberger RE (Feb 2002). "Cardiac troponin T lysine 210 deletion in a family with dilated cardiomyopathy". Journal of Cardiac Failure. 8 (1): 28–32. doi:10.1054/jcaf.2002.31157. PMID 11862580.
- Hershberger RE, Pinto JR, Parks SB, Kushner JD, Li D, Ludwigsen S, Cowan J, Morales A, Parvatiyar MS, Potter JD (Aug 2009). "Clinical and functional characterization of TNNT2 mutations identified in patients with dilated cardiomyopathy". Circulation: Cardiovascular Genetics. 2 (4): 306–13. doi:10.1161/CIRCGENETICS.108.846733. PMC 2900844. PMID 20031601.
- Morimoto S, Nakaura H, Yanaga F, Ohtsuki I (Jul 1999). "Functional consequences of a carboxyl terminal missense mutation Arg278Cys in human cardiac troponin T". Biochemical and Biophysical Research Communications. 261 (1): 79–82. doi:10.1006/bbrc.1999.1000. PMID 10405326.
External links
- Mass spectrometry characterization of human TNNT2 at COPaKB Archived 2016-03-05 at the Wayback Machine
- GeneReviews/NIH/NCBI/UW entry on Familial Hypertrophic Cardiomyopathy Overview
- Overview of all the structural information available in the PDB for UniProt: P45379 (Troponin T, cardiac muscle) at the PDBe-KB.