Pharmacokinetics of testosterone
The pharmacology of testosterone, an androgen and anabolic steroid (AAS) medication and naturally occurring steroid hormone, concerns its pharmacodynamics, pharmacokinetics, and various routes of administration.
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral, buccal, sublingual, intranasal, transdermal (gel, cream, patch, solution), vaginal (cream, gel, suppository), rectal (suppository), intramuscular or subcutaneous injection (oil solution, aqueous suspension), subcutaneous implant (pellet) |
| Drug class | Androgen, anabolic steroid |
| Pharmacokinetic data | |
| Bioavailability | Oral: very low (due to extensive first pass metabolism) |
| Protein binding | 97.0–99.5% (to SHBGTooltip sex hormone-binding globulin and albumin)[1] |
| Metabolism | Liver (mainly reduction and conjugation) |
| Elimination half-life | 2–4 hours |
| Excretion | Urine (90%), feces (6%) |
Testosterone is a naturally occurring and bioidentical AAS, or an agonist of the androgen receptor, the biological target of androgens like endogenous testosterone and dihydrotestosterone (DHT).
Testosterone is used by both men and women and can be taken by a variety of different routes of administration.[2]
Routes of administration
Testosterone can be taken by a variety of different routes of administration.[2][3] These include oral, buccal, sublingual, intranasal, transdermal (gels, creams, patches, solutions), vaginal (creams, gels, suppositories), rectal (suppositories), by intramuscular or subcutaneous injection (in oil solutions or aqueous suspensions), and as a subcutaneous implant.[2][3] The pharmacokinetics of testosterone, including its bioavailability, metabolism, biological half-life, and other parameters, differ by route of administration.[2] Likewise, the potency of testosterone, and its local effects in certain tissues, for instance the liver, differ by route of administration as well.[2] In particular, the oral route is subject to a high first-pass effect, which results in high levels of testosterone in the liver and consequent hepatic androgenic effects, as well as low potency due to first-pass metabolism in the intestines and liver into metabolites like dihydrotestosterone and androgen conjugates.[2] Conversely, this is not the case for non-oral routes, which bypass the first pass.[2]
Different testosterone routes and dosages can achieve widely varying circulating testosterone levels.[2] For purposes of comparison with normal physiological circumstances, circulating levels of total testosterone in men range from about 250 to 1,100 ng/dL (mean 630 ng/dL) and in women range from about 2 to 50 ng/dL (mean 32 ng/dL).[4][5][6][7] Testosterone levels decline with age in men.[8] In women with polycystic ovary syndrome (PCOS), a condition of androgen excess, testosterone levels are typically around 50 to 80 ng/dL, with a range of about 30 to 140 ng/dL.[9][10][7] Total testosterone levels are about 20-fold and free testosterone levels about 40-fold higher in men than in women on average.[11] Similarly, testosterone production is approximately 30 times higher in men than in women.[12]
| Route | Ingredient | Form | Dose[lower-alpha 2] | Brand names[lower-alpha 3] | |
|---|---|---|---|---|---|
| Oral | Test. undecanoate | Capsule | 40 mg | Andriol, Jatenzo | |
| Sublingual | Testosterone | Tablet | 10 mg | Testoral | |
| Buccal | Testosterone | Tablet | 30 mg | Striant | |
| Intranasal | Testosterone | Nasal gel | 5.5 mg/spray, 120 sprays | Natesto | |
| Transdermal | Testosterone | Non-scrotal patch | 2.5, 4, 5, 6 mg/day | Androderm | |
| Non-scrotal patch | 150, 300 μg/day | Intrinsa | |||
| Scrotal patch[lower-alpha 4] | 4, 6 mg/day | Testoderm | |||
| Topical gel | 25, 50, 75, 100, 125 mg/pump | AndroGel, Testim | |||
| Axillary solution | 30 mg/pump | Axiron | |||
| Rectal | Testosterone | Suppository | 40 mg | Rektandron | |
| Injection[lower-alpha 5] | Test. enanthate | Oil solution | 50, 100, 180, 200, 250 mg/mL | Delatestryl | |
| Test. cypionate | Oil solution | 50, 100, 200, 250 mg/mL | Depo-Testosterone | ||
| Mixed test. esters[lower-alpha 6] | Oil solution | 100, 250 mg/mL | Sustanon | ||
| Test. undecanoate | Oil solution | 750, 1000 mg | Aveed, Nebido | ||
| Implant | Testosterone | Pellet | 50, 75, 100, 200 mg | Testopel | |
Footnotes and sources:
| |||||
| Route | Medication | Major brand names | Form | Dosage |
|---|---|---|---|---|
| Oral | Testosteronea | – | Tablet | 400–800 mg/day (in divided doses) |
| Testosterone undecanoate | Andriol, Jatenzo | Capsule | 40–80 mg/2–4x day (with meals) | |
| Methyltestosteroneb | Android, Metandren, Testred | Tablet | 10–50 mg/day | |
| Fluoxymesteroneb | Halotestin, Ora-Testryl, Ultandren | Tablet | 5–20 mg/day | |
| Metandienoneb | Dianabol | Tablet | 5–15 mg/day | |
| Mesteroloneb | Proviron | Tablet | 25–150 mg/day | |
| Sublingual | Testosteroneb | Testoral | Tablet | 5–10 mg 1–4x/day |
| Methyltestosteroneb | Metandren, Oreton Methyl | Tablet | 10–30 mg/day | |
| Buccal | Testosterone | Striant | Tablet | 30 mg 2x/day |
| Methyltestosteroneb | Metandren, Oreton Methyl | Tablet | 5–25 mg/day | |
| Transdermal | Testosterone | AndroGel, Testim, TestoGel | Gel | 25–125 mg/day |
| Androderm, AndroPatch, TestoPatch | Non-scrotal patch | 2.5–15 mg/day | ||
| Testoderm | Scrotal patch | 4–6 mg/day | ||
| Axiron | Axillary solution | 30–120 mg/day | ||
| Androstanolone (DHT) | Andractim | Gel | 100–250 mg/day | |
| Rectal | Testosterone | Rektandron, Testosteronb | Suppository | 40 mg 2–3x/day |
| Injection (IMTooltip intramuscular injection or SCTooltip subcutaneous injection) | Testosterone | Andronaq, Sterotate, Virosterone | Aqueous suspension | 10–50 mg 2–3x/week |
| Testosterone propionateb | Testoviron | Oil solution | 10–50 mg 2–3x/week | |
| Testosterone enanthate | Delatestryl | Oil solution | 50–250 mg 1x/1–4 weeks | |
| Xyosted | Auto-injector | 50–100 mg 1x/week | ||
| Testosterone cypionate | Depo-Testosterone | Oil solution | 50–250 mg 1x/1–4 weeks | |
| Testosterone isobutyrate | Agovirin Depot | Aqueous suspension | 50–100 mg 1x/1–2 weeks | |
| Testosterone phenylacetateb | Perandren, Androject | Oil solution | 50–200 mg 1x/3–5 weeks | |
| Mixed testosterone esters | Sustanon 100, Sustanon 250 | Oil solution | 50–250 mg 1x/2–4 weeks | |
| Testosterone undecanoate | Aveed, Nebido | Oil solution | 750–1,000 mg 1x/10–14 weeks | |
| Testosterone buciclatea | – | Aqueous suspension | 600–1,000 mg 1x/12–20 weeks | |
| Implant | Testosterone | Testopel | Pellet | 150–1,200 mg/3–6 months |
| Notes: Men produce about 3 to 11 mg testosterone per day (mean 7 mg/day in young men). Footnotes: a = Never marketed. b = No longer used and/or no longer marketed. Sources: See template. | ||||
| Route | Medication | Major brand names | Form | Dosage |
|---|---|---|---|---|
| Oral | Testosterone undecanoate | Andriol, Jatenzo | Capsule | 40–80 mg 1x/1–2 days |
| Methyltestosterone | Metandren, Estratest | Tablet | 0.5–10 mg/day | |
| Fluoxymesterone | Halotestin | Tablet | 1–2.5 mg 1x/1–2 days | |
| Normethandronea | Ginecoside | Tablet | 5 mg/day | |
| Tibolone | Livial | Tablet | 1.25–2.5 mg/day | |
| Prasterone (DHEA)b | – | Tablet | 10–100 mg/day | |
| Sublingual | Methyltestosterone | Metandren | Tablet | 0.25 mg/day |
| Transdermal | Testosterone | Intrinsa | Patch | 150–300 μg/day |
| AndroGel | Gel, cream | 1–10 mg/day | ||
| Vaginal | Prasterone (DHEA) | Intrarosa | Insert | 6.5 mg/day |
| Injection | Testosterone propionatea | Testoviron | Oil solution | 25 mg 1x/1–2 weeks |
| Testosterone enanthate | Delatestryl, Primodian Depot | Oil solution | 25–100 mg 1x/4–6 weeks | |
| Testosterone cypionate | Depo-Testosterone, Depo-Testadiol | Oil solution | 25–100 mg 1x/4–6 weeks | |
| Testosterone isobutyratea | Femandren M, Folivirin | Aqueous suspension | 25–50 mg 1x/4–6 weeks | |
| Mixed testosterone esters | Climacterona | Oil solution | 150 mg 1x/4–8 weeks | |
| Omnadren, Sustanon | Oil solution | 50–100 mg 1x/4–6 weeks | ||
| Nandrolone decanoate | Deca-Durabolin | Oil solution | 25–50 mg 1x/6–12 weeks | |
| Prasterone enanthatea | Gynodian Depot | Oil solution | 200 mg 1x/4–6 weeks | |
| Implant | Testosterone | Testopel | Pellet | 50–100 mg 1x/3–6 months |
| Notes: Premenopausal women produce about 230 ± 70 μg testosterone per day (6.4 ± 2.0 mg testosterone per 4 weeks), with a range of 130 to 330 μg per day (3.6–9.2 mg per 4 weeks). Footnotes: a = Mostly discontinued or unavailable. b = Over-the-counter. Sources: See template. | ||||
| Total testosterone | |||||
|---|---|---|---|---|---|
| Stage | Age range | Male | Female | ||
| Values | SI units | Values | SI units | ||
| Infant | Premature (26–28 weeks) | 59–125 ng/dL | 2.047–4.337 nmol/L | 5–16 ng/dL | 0.173–0.555 nmol/L |
| Premature (31–35 weeks) | 37–198 ng/dL | 1.284–6.871 nmol/L | 5–22 ng/dL | 0.173–0.763 nmol/L | |
| Newborn | 75–400 ng/dL | 2.602–13.877 nmol/L | 20–64 ng/dL | 0.694–2.220 nmol/L | |
| Child | 1–6 years | ND | ND | ND | ND |
| 7–9 years | 0–8 ng/dL | 0–0.277 nmol/L | 1–12 ng/dL | 0.035–0.416 nmol/L | |
| Just before puberty | 3–10 ng/dL* | 0.104–0.347 nmol/L* | <10 ng/dL* | <0.347 nmol/L* | |
| Puberty | 10–11 years | 1–48 ng/dL | 0.035–1.666 nmol/L | 2–35 ng/dL | 0.069–1.214 nmol/L |
| 12–13 years | 5–619 ng/dL | 0.173–21.480 nmol/L | 5–53 ng/dL | 0.173–1.839 nmol/L | |
| 14–15 years | 100–320 ng/dL | 3.47–11.10 nmol/L | 8–41 ng/dL | 0.278–1.423 nmol/L | |
| 16–17 years | 200–970 ng/dL* | 6.94–33.66 nmol/L* | 8–53 ng/dL | 0.278–1.839 nmol/L | |
| Adult | ≥18 years | 350–1080 ng/dL* | 12.15–37.48 nmol/L* | – | – |
| 20–39 years | 400–1080 ng/dL | 13.88–37.48 nmol/L | – | – | |
| 40–59 years | 350–890 ng/dL | 12.15–30.88 nmol/L | – | – | |
| ≥60 years | 350–720 ng/dL | 12.15–24.98 nmol/L | – | – | |
| Premenopausal | – | – | 10–54 ng/dL | 0.347–1.873 nmol/L | |
| Postmenopausal | – | – | 7–40 ng/dL | 0.243–1.388 nmol/L | |
| Bioavailable testosterone | |||||
| Stage | Age range | Male | Female | ||
| Values | SI units | Values | SI units | ||
| Child | 1–6 years | 0.2–1.3 ng/dL | 0.007–0.045 nmol/L | 0.2–1.3 ng/dL | 0.007–0.045 nmol/L |
| 7–9 years | 0.2–2.3 ng/dL | 0.007–0.079 nmol/L | 0.2–4.2 ng/dL | 0.007–0.146 nmol/L | |
| Puberty | 10–11 years | 0.2–14.8 ng/dL | 0.007–0.513 nmol/L | 0.4–19.3 ng/dL | 0.014–0.670 nmol/L |
| 12–13 years | 0.3–232.8 ng/dL | 0.010–8.082 nmol/L | 1.1–15.6 ng/dL | 0.038–0.541 nmol/L | |
| 14–15 years | 7.9–274.5 ng/dL | 0.274–9.525 nmol/L | 2.5–18.8 ng/dL | 0.087–0.652 nmol/L | |
| 16–17 years | 24.1–416.5 ng/dL | 0.836–14.452 nmol/L | 2.7–23.8 ng/dL | 0.094–0.826 nmol/L | |
| Adult | ≥18 years | ND | ND | – | – |
| Premenopausal | – | – | 1.9–22.8 ng/dL | 0.066–0.791 nmol/L | |
| Postmenopausal | – | – | 1.6–19.1 ng/dL | 0.055–0.662 nmol/L | |
| Free testosterone | |||||
| Stage | Age range | Male | Female | ||
| Values | SI units | Values | SI units | ||
| Child | 1–6 years | 0.1–0.6 pg/mL | 0.3–2.1 pmol/L | 0.1–0.6 pg/mL | 0.3–2.1 pmol/L |
| 7–9 years | 0.1–0.8 pg/mL | 0.3–2.8 pmol/L | 0.1–1.6 pg/mL | 0.3–5.6 pmol/L | |
| Puberty | 10–11 years | 0.1–5.2 pg/mL | 0.3–18.0 pmol/L | 0.1–2.9 pg/mL | 0.3–10.1 pmol/L |
| 12–13 years | 0.4–79.6 pg/mL | 1.4–276.2 pmol/L | 0.6–5.6 pg/mL | 2.1–19.4 pmol/L | |
| 14–15 years | 2.7–112.3 pg/mL | 9.4–389.7 pmol/L | 1.0–6.2 pg/mL | 3.5–21.5 pmol/L | |
| 16–17 years | 31.5–159 pg/mL | 109.3–551.7 pmol/L | 1.0–8.3 pg/mL | 3.5–28.8 pmol/L | |
| Adult | ≥18 years | 44–244 pg/mL | 153–847 pmol/L | – | – |
| Premenopausal | – | – | 0.8–9.2 pg/mL | 2.8–31.9 pmol/L | |
| Postmenopausal | – | – | 0.6–6.7 pg/mL | 2.1–23.2 pmol/L | |
| Sources: See template. | |||||
Oral testosterone
Testosterone is well-absorbed but extensively metabolized with oral administration due to the first pass through the intestines and liver.[2][27][28][3] It is rapidly and completely inactivated in men at doses of less than 200 mg.[2][27] In large doses, such as 200 mg however, significant increases in circulating testosterone levels become apparent.[2][27] In addition, while a 60 mg dose has no effect on testosterone levels in men, this dose does measurably increase testosterone levels in prepubertal boys and women.[27] The oral bioavailability of testosterone in young women after a single 25 mg dose was found to be 3.6 ± 2.5%.[29] High levels of testosterone are also achieved with a 60 mg dose of oral testosterone in men with liver cirrhosis.[2] These findings are attributed to induction of liver enzymes by testosterone and consequent activation of its own metabolism.[2][27] Substitution dosages of oral testosterone in men are in the range of 400 to 800 mg/day.[27][28] Such doses exceed the amount of testosterone produced by the body, which is approximately 7 mg/day, by approximately 100-fold.[2][27][28] The elimination half-life of oral testosterone is rapid at about 5 to 7 hours.[28][30] As a result, it requires administration several times per day in divided doses.[28] Due to its limitations, such as the high doses required and necessity of multiple daily doses, oral testosterone is not used clinically in its unmodified form.[28][3]
Oral testosterone has been studied in combination with a 5α-reductase inhibitor to reduce its first-pass metabolism and improve its bioavailability.[2][31]
Oral testosterone undecanoate
Instead of in its free unesterified form, testosterone is used by oral administration in the form of testosterone undecanoate.[2] Due to the unique chemical properties afforded by its long fatty acid ester chain, this testosterone ester is partially absorbed from the gastrointestinal tract into the lymphatic system, thereby bypassing a portion of first-pass metabolism in the liver and producing measurable increases in testosterone levels at much lower doses than free testosterone.[2][3] Of oral testosterone undecanoate that reaches circulation, 90 to 100% is transported lymphatically.[32] However, its duration remains short, with an elimination half-life of 1.6 hours and a mean residence time of 3.7 hours.[33][34][35] Oral testosterone undecanoate is provided as 40 mg oil-filled capsules and requires administration 2 to 4 times per day (i.e., 80 to 160 mg/day) for substitution in men.[2][33][3] It must be taken with food containing at least a moderate or "normal" amount of fat in order to achieve adequate absorption.[2][36][37][38] In addition, there is very high interindividual variability in levels of testosterone with oral testosterone undecanoate.[39] The bioavailability of oral testosterone undecanoate taken with food is 3 to 7%.[32][40] Inappropriately high levels of testosterone have been observed with 10 to 40 mg/day oral testosterone undecanoate in women.[41][42] The oral bioavailability of testosterone undecanoate in young women after a single 40 mg dose was found to be 6.8 ± 3.3%.[29]
A novel self-emulsifying formulation of oral testosterone undecanoate in 300-mg capsules for use once per day is under development.[39]
First-pass effect and differences
Oral testosterone and oral testosterone undecanoate are not hepatotoxic, unlike orally administered 17α-alkylated anabolic steroids such as methyltestosterone and fluoxymesterone but similarly to parenteral routes and forms of bioidentical testosterone like injections.[43][2][39]
Buccal administration
Testosterone can be used by buccal administration (e.g., brand name Striant).[2]
Sublingual administration
Testosterone can be used by sublingual administration.[2][44][45] A 10 mg sublingual tablet with the brand name Testoral was previously marketed for use one to four times per day in men.[46]
Inhalational administration
Testosterone has been studied by inhalation.[47]
Intranasal administration
Testosterone can be used by intranasal administration (e.g., brand name Natesto).[2]
Transdermal administration
Testosterone is available for transdermal administration in the form of gels, creams, scrotal and non-scrotal patches, and axillary solutions.[2]
Transdermal testosterone gel has a bioavailability of about 8 to 14% when administered to recommended skin sites including the abdomen, arms, shoulders, and thighs.[48][49] Scrotal skin is the thinnest skin of the body[50] and has enhanced absorption characteristics relative to other skin areas.[51][52][53][54] Application of testosterone gels and creams to the scrotum has been studied and achieves much higher levels of testosterone than conventional skin sites.[55][56][57][58] Scrotal application of testosterone requires approximately 5-fold lower doses relative to non-scrotal application.[59][50]
The development of transdermal preparations of testosterone (and of progesterone)[60] has been more difficult than the case of estradiol.[50] This is because testosterone levels in men are about 100 to 1,000 times higher than estradiol levels in women (300 to 1,000 ng/dL vs. 50 to 150 pg/mL, respectively).[50] Non-scrotal testosterone patches were assessed and were found to be ineffective in raising testosterone levels in men.[50] As a result, scrotal testosterone patches were initially marketed.[50] Subsequently, however, non-scrotal testosterone patches with special permeation enhancers that could successfully increase testosterone levels were developed and marketed.[50] However, non-scrotal testosterone patches nonetheless require a large skin area for application (up to 60 cm2) and must be replaced daily.[50]
Supraphysiological levels of dihydrotestosterone (DHT) occur with scrotal application of testosterone, whereas this does not occur with non-scrotal transdermal application.[50] This is due to the high expression of 5α-reductase in scrotal skin.[50] Estradiol levels are similar with scrotal versus non-scrotal application of transdermal testosterone.[50]
Low-dose transdermal testosterone patches in women have been found to result in testosterone levels of 64 ng/dL with 150 μg/day and 102 ng/dL with 300 μg/day.[41] When testosterone is used transdermally in women or transmen, hair growth at the application sites can happen.[61]
Vaginal administration
Testosterone can be used by vaginal administration of creams, suppositories, and vaginal rings available from compounding pharmacies.[62][63][64][65][66][67]
Rectal administration
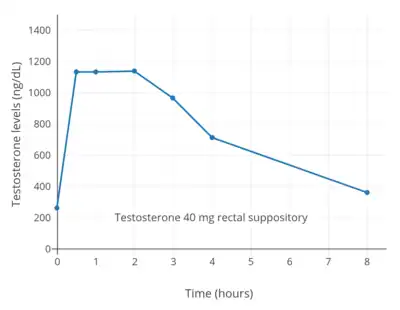
Testosterone was marketed as a suppository for rectal administration by Ferring Pharmaceuticals from the early 1960s under brand names such as Rektandron and Testosteron.[43][25][26] Rectal administration of testosterone avoids the first-pass effect with oral administration similarly to other non-oral routes.[2] A single 40 mg dose of rectal testosterone has been found to result in maximal testosterone levels of almost 1,200 ng/dL within 30 minutes.[27] Subsequently, testosterone levels steadily decline, reaching levels of about 700 ng/dL after 4 hours and levels of about 400 ng/dL after 8 hours.[27] Other studies have also assessed the use of rectal testosterone, with similar findings.[2][68][69][70][71][72] Rectal use of testosterone requires administration two or three times per day to maintain adequate testosterone levels.[27][2] The route is poorly accepted, owing to its inconvenience.[2] Rectal testosterone has been used in transmasculine hormone therapy.[69]
Intramuscular injection
Testosterone can be administered by intramuscular injection either as an aqueous suspension of testosterone or as an oil solution or aqueous suspension of testosterone esters such as testosterone propionate, testosterone enanthate, testosterone cypionate, testosterone undecanoate, and testosterone isobutyrate.[2][35][3] An even longer-acting testosterone ester that was developed but ultimately never marketed is testosterone buciclate.[3] These preparations are prodrugs of progesterone that have a long-lasting depot effect when injected into muscle or fat, ranging from days to months in duration.[2]
The bioavailability of drugs that are administered intramuscularly is generally almost 95%.[73]
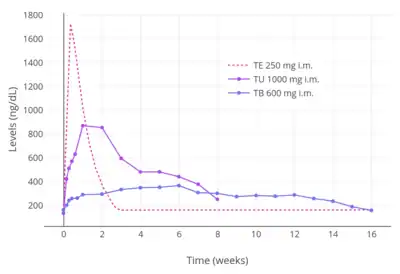
As oil solutions by intramuscular injection, the elimination half-lives of testosterone esters are 0.8 days for testosterone propionate, 4.5 days for testosterone enanthate, 20.9 days (in tea seed oil) and 33.9 days (in caster oil) for testosterone undecanoate, and 29.5 days for testosterone buciclate.[8][33] The pharmacokinetics of testosterone cypionate are said to be the same as those of testosterone enanthate, with "extremely comparable" patterns of testosterone release.[35][33] Due to their varying and different elimination half-lives, the different intramuscular testosterone esters are administered with differing frequencies.[74] Testosterone propionate is injected two to three times per week, testosterone enanthate and testosterone cypionate are injected once every two to four weeks, and testosterone undecanoate and testosterone buciclate are injected once every 10 to 14 weeks.[74] Due to its relatively short duration, testosterone propionate is now relatively little used and testosterone undecanoate is the preferred testosterone ester for intramuscular use.[8][33] Testosterone undecanoate and testosterone buciclate can be injected intramuscularly as infrequently as four times per year.[8][33]
High doses of testosterone esters by intramuscular injection have been studied in healthy young men.[75] Levels of testosterone with intramuscular injections of testosterone cypionate were about 700 ng/dL for 100 mg/week, 1100 ng/dL for 250 mg/week, and 2000 ng/dL for 500 mg/week.[75][76] In another study, testosterone levels with 600 mg/week testosterone enanthate by intramuscular injection were 2,800–3,200 ng/dL.[75][77]
Intramuscular injection of testosterone propionate as an oil solution, aqueous suspension, and emulsion has been compared.[78]
Intramuscular injection of testosterone-containing biodegradable microspheres has been studied.[2]
| Androgen | Structure | Ester | Relative mol. weight | Relative T contentb | logPc | ||||
|---|---|---|---|---|---|---|---|---|---|
| Position(s) | Moiet(ies) | Type | Lengtha | ||||||
| Testosterone | – | – | – | – | 1.00 | 1.00 | 3.0–3.4 | ||
| Testosterone propionate | C17β | Propanoic acid | Straight-chain fatty acid | 3 | 1.19 | 0.84 | 3.7–4.9 | ||
| Testosterone isobutyrate | C17β | Isobutyric acid | Branched-chain fatty acid | – (~3) | 1.24 | 0.80 | 4.9–5.3 | ||
| Testosterone isocaproate | C17β | Isohexanoic acid | Branched-chain fatty acid | – (~5) | 1.34 | 0.75 | 4.4–6.3 | ||
| Testosterone caproate | C17β | Hexanoic acid | Straight-chain fatty acid | 6 | 1.35 | 0.75 | 5.8–6.5 | ||
| Testosterone phenylpropionate | C17β | Phenylpropanoic acid | Aromatic fatty acid | – (~6) | 1.46 | 0.69 | 5.8–6.5 | ||
| Testosterone cypionate | C17β | Cyclopentylpropanoic acid | Cyclic carboxylic acid | – (~6) | 1.43 | 0.70 | 5.1–7.0 | ||
| Testosterone enanthate | C17β | Heptanoic acid | Straight-chain fatty acid | 7 | 1.39 | 0.72 | 3.6–7.0 | ||
| Testosterone decanoate | C17β | Decanoic acid | Straight-chain fatty acid | 10 | 1.53 | 0.65 | 6.3–8.6 | ||
| Testosterone undecanoate | C17β | Undecanoic acid | Straight-chain fatty acid | 11 | 1.58 | 0.63 | 6.7–9.2 | ||
| Testosterone buciclated | C17β | Bucyclic acide | Cyclic carboxylic acid | – (~9) | 1.58 | 0.63 | 7.9–8.5 | ||
| Footnotes: a = Length of ester in carbon atoms for straight-chain fatty acids or approximate length of ester in carbon atoms for aromatic or cyclic fatty acids. b = Relative testosterone content by weight (i.e., relative androgenic/anabolic potency). c = Experimental or predicted octanol/water partition coefficient (i.e., lipophilicity/hydrophobicity). Retrieved from PubChem, ChemSpider, and DrugBank. d = Never marketed. e = Bucyclic acid = trans-4-Butylcyclohexane-1-carboxylic acid. Sources: See individual articles. | |||||||||
| Testosterone ester | Form | Route | TmaxTooltip Time to peak levels | t1/2Tooltip Elimination half-life | MRTTooltip Mean residence time |
|---|---|---|---|---|---|
| Testosterone undecanoate | Oil-filled capsules | Oral | ? | 1.6 hours | 3.7 hours |
| Testosterone propionate | Oil solution | Intramuscular injection | ? | 0.8 days | 1.5 days |
| Testosterone enanthate | Castor oil solution | Intramuscular injection | 10 days | 4.5 days | 8.5 days |
| Testosterone undecanoate | Tea seed oil solution | Intramuscular injection | 13.0 days | 20.9 days | 34.9 days |
| Testosterone undecanoate | Castor oil solution | Intramuscular injection | 11.4 days | 33.9 days | 36.0 days |
| Testosterone buciclatea | Aqueous suspension | Intramuscular injection | 25.8 days | 29.5 days | 60.0 days |
| Notes: Testosterone cypionate has similar pharmacokinetics to Testosterone enanthate. Footnotes: a = Never marketed. Sources: See template. | |||||
| Medication | Form | Major brand names | Duration |
|---|---|---|---|
| Testosterone | Aqueous suspension | Andronaq, Sterotate, Virosterone | 2–3 days |
| Testosterone propionate | Oil solution | Androteston, Perandren, Testoviron | 3–4 days |
| Testosterone phenylpropionate | Oil solution | Testolent | 8 days |
| Testosterone isobutyrate | Aqueous suspension | Agovirin Depot, Perandren M | 14 days |
| Mixed testosterone estersa | Oil solution | Triolandren | 10–20 days |
| Mixed testosterone estersb | Oil solution | Testosid Depot | 14–20 days |
| Testosterone enanthate | Oil solution | Delatestryl | 14–28 days |
| Testosterone cypionate | Oil solution | Depovirin | 14–28 days |
| Mixed testosterone estersc | Oil solution | Sustanon 250 | 28 days |
| Testosterone undecanoate | Oil solution | Aveed, Nebido | 100 days |
| Testosterone buciclated | Aqueous suspension | 20 Aet-1, CDB-1781e | 90–120 days |
| Nandrolone phenylpropionate | Oil solution | Durabolin | 10 days |
| Nandrolone decanoate | Oil solution | Deca Durabolin | 21–28 days |
| Methandriol | Aqueous suspension | Notandron, Protandren | 8 days |
| Methandriol bisenanthoyl acetate | Oil solution | Notandron Depot | 16 days |
| Metenolone acetate | Oil solution | Primobolan | 3 days |
| Metenolone enanthate | Oil solution | Primobolan Depot | 14 days |
| Note: All are via i.m. injection. Footnotes: a = TP, TV, and TUe. b = TP and TKL. c = TP, TPP, TiCa, and TD. d = Studied but never marketed. e = Developmental code names. Sources: See template. | |||
Subcutaneous injection
Testosterone esters like testosterone enanthate and testosterone cypionate can be given by subcutaneous injection instead of intramuscular injection. Studies have shown that subcutaneous injection of testosterone and closely related esters in oil like testosterone cypionate, testosterone enantate, and nandrolone decanoate is effective and has similar pharmacokinetics to intramuscular injection.[79][80][81][82][83][84][85]
Subcutaneous implant
Testosterone can be administered in the form of a subcutaneous pellet implant.[2]
The bioavailability of testosterone when administered as a subcutaneous pellet implant is virtually 100%.[86] Levels of testosterone vary considerably between individuals, but are fairly constant within individuals.[41] The absorption half-life of subdermal testosterone implants is 2.5 months.[8] The replacement interval is once every four to six months.[41][87] A single 50 mg testosterone pellet implanted every 4 to 6 months has been found to result in testosterone levels of 70 to 90 ng/dL in women.[41]
Intravenous injection
Testosterone esters like testosterone enanthate are hydrolyzed into testosterone so rapidly in the blood that testosterone and testosterone enanthate have nearly identical pharmacokinetics when administered via intravenous injection.[2]
General
Absorption
The oral bioavailability of testosterone is very low.[8][88] The bioavailability of oral testosterone undecanoate is 3 to 7%.[32][40] Topical testosterone gels have a bioavailability of about 8 to 14% when administered to recommended skin sites including the abdomen, arms, shoulders, and thighs.[48][49] The bioavailability of testosterone by subcutaneous implant is virtually 100%.[86] The bioavailability of drugs that are administered intramuscularly is generally almost 95%.[73]
Distribution
In the circulation, 97.0 to 99.5% of testosterone is bound to plasma proteins, with 0.5 to 3.0% unbound.[1] It is tightly bound to SHBG and weakly to albumin.[1] Of circulating testosterone, 30 to 44% is bound to SHBG while 54 to 68% is bound to albumin.[1] Testosterone that is unbound is referred to as free testosterone and testosterone that is bound to albumin is referred to as bioavailable testosterone.[1] Unlike testosterone that is bound to SHBG, bioavailable testosterone is bound to plasma proteins weakly enough such that, similarly to free testosterone, it may be biologically active, at least to a certain extent.[1] When referenced collectively (i.e., free, bioavailable, and SHBG-bound), circulating testosterone is referred to as total testosterone.[1]
Metabolism
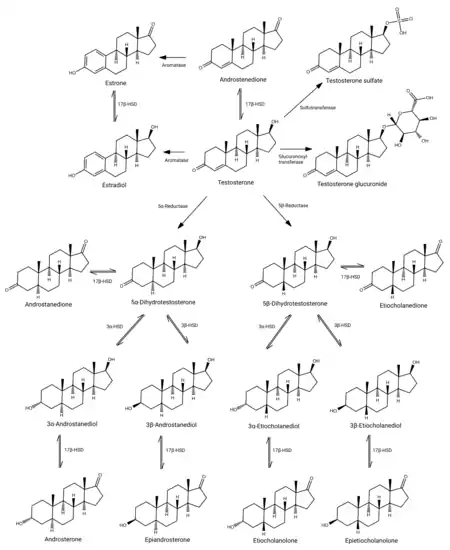
Testosterone metabolism in humans
|
Testosterone is metabolized primarily in the liver mainly (90%) by reduction via 5α- and 5β-reductase and conjugation via glucuronidation and sulfation.[1][89][90] The major urinary metabolites of testosterone are androsterone glucuronide and etiocholanolone glucuronide.[1][89][90][91]
The elimination half-life of testosterone varies depending on the route of administration and formulation and on whether or not it is esterified.[8] The elimination half-life of testosterone in the blood or by intravenous injection is only about 10 minutes.[8][33] Conversely, testosterone and testosterone esters in oil solution or crystalline aqueous suspension administered by intramuscular or subcutaneous injection have much longer half-lives, in the range of days to months, due to slow release from the injection site.[8][33]
Elimination
Testosterone and its metabolites are eliminated in urine.[92] It is excreted mainly as androsterone glucuronide and etiocholanolone glucuronide.[91] It is also excreted to a small extent as other conjugates such as testosterone glucuronide (1%), testosterone sulfate (0.03%), and androstanediol glucuronides.[91][93] Only a very small amount of testosterone (less than 0.01%) is found unchanged in the urine.[92][93]
See also
References
- Melmed S, Polonsky KS, Larsen PR, Kronenberg HM (11 November 2015). Williams Textbook of Endocrinology. Elsevier Health Sciences. pp. 709, 711, 765. ISBN 978-0-323-34157-8.
- Behre, Hermann M.; Nieschlag, Eberhard; Nieschlag, Eberhard; Behre, Hermann M.; Nieschlag, Susan (26 July 2012). "Testosterone preparations for clinical use in males". In Eberhard Nieschlag; Hermann M. Behre; Susan Nieschlag (eds.). Testosterone: Action, Deficiency, Substitution. Cambridge University Press. pp. 309–335. doi:10.1017/CBO9781139003353.016. ISBN 978-1-107-01290-5.
- Becker KL (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 1116, 1119, 1152, 1182–1185, 1195–1197, 2146. ISBN 978-0-7817-1750-2. Archived from the original on May 5, 2017.
- J. Larry Jameson; Leslie J. De Groot (25 February 2015). Endocrinology: Adult and Pediatric E-Book. Elsevier Health Sciences. ISBN 978-0-323-32195-2.
- Chernecky CC, Berger BJ (31 October 2012). Laboratory Tests and Diagnostic Procedures – E-Book. Elsevier Health Sciences. pp. 1059–1062. ISBN 978-1-4557-4502-9.
- Mark A. Sperling (10 April 2014). Pediatric Endocrinology E-Book: Expert Consult - Online and Print. Elsevier Health Sciences. pp. 488–. ISBN 978-1-4557-5973-6.
- Steinberger, Emil; Ayala, Carma; Hsi, Bartholomew; Smith, Keith D.; Rodriguez-Rigau, Luis J.; Weidman, E. Russell; Reimondo, Gerold G. (1998). "Utilization of commercial laboratory results in management of hyperandrogenism in women". Endocrine Practice. 4 (1): 1–10. doi:10.4158/EP.4.1.1. ISSN 1530-891X. PMID 15251757.
- Nieschlag E, Behre HM (6 December 2012). Testosterone: Action - Deficiency - Substitution. Springer Science & Business Media. pp. 1–, 9, 298, 309–331, 349–353, 366–367. ISBN 978-3-642-72185-4.
- Legro, Richard S.; Schlaff, William D.; Diamond, Michael P.; Coutifaris, Christos; Casson, Peter R.; Brzyski, Robert G.; Christman, Gregory M.; Trussell, J. C.; Krawetz, Stephen A.; Snyder, Peter J.; Ohl, Dana; Carson, Sandra A.; Steinkampf, Michael P.; Carr, Bruce R.; McGovern, Peter G.; Cataldo, Nicholas A.; Gosman, Gabriella G.; Nestler, John E.; Myers, Evan R.; Santoro, Nanette; Eisenberg, Esther; Zhang, Meizhuo; Zhang, Heping (2010). "Total Testosterone Assays in Women with Polycystic Ovary Syndrome: Precision and Correlation with Hirsutism". The Journal of Clinical Endocrinology & Metabolism. 95 (12): 5305–5313. doi:10.1210/jc.2010-1123. ISSN 0021-972X. PMC 2999971. PMID 20826578.
- Balen, Adam H.; Conway, Gerry S.; Kaltsas, Gregory; Techatraisak, Kitirak; Manning, Patrick J.; West, Christine; Jacobs, Howard S. (1995). "Andrology: Polycystic ovary syndrome: the spectrum of the disorder in 1741 patients". Human Reproduction. 10 (8): 2107–2111. doi:10.1093/oxfordjournals.humrep.a136243. ISSN 1460-2350. PMID 8567849.
- Styne, D. M. (6 December 2019). "Physiology and Disorders of Puberty". In Melmed, S.; Koenig, R. J.; Rosen, C. J.; Auchus, R. J.; Goldfine, A. B.; Williams, R. H. (eds.). Williams Textbook of Endocrinology (14 ed.). Philadelphia, PA: Elsevier. pp. 1023–1164. ISBN 9780323555968.
- Liu, Peter Y.; Handelsman, David J. (1998). "Androgen therapy in non-gonadal disease". Testosterone. pp. 473–512. doi:10.1007/978-3-642-72185-4_17. ISBN 978-3-642-72187-8.
- Nieschlag E (September 2006). "Testosterone treatment comes of age: new options for hypogonadal men". Clin. Endocrinol. (Oxf). 65 (3): 275–81. doi:10.1111/j.1365-2265.2006.02618.x. PMID 16918944.
- Nieschlag E (January 2015). "Current topics in testosterone replacement of hypogonadal men". Best Pract. Res. Clin. Endocrinol. Metab. 29 (1): 77–90. doi:10.1016/j.beem.2014.09.008. PMID 25617174.
- Byrne M, Nieschlag E (May 2003). "Testosterone replacement therapy in male hypogonadism". J. Endocrinol. Invest. 26 (5): 481–9. doi:10.1007/bf03345206. PMID 12906378.
- Cappa M, Cianfarani S, Ghizzoni L, Loche S, Maghnie M (10 December 2015). Advanced Therapies in Pediatric Endocrinology and Diabetology: Workshop, Rome, October 2014. Karger Medical and Scientific Publishers. pp. 68–. ISBN 978-3-318-05637-2.
- "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. Archived from the original on November 16, 2016. Retrieved November 16, 2016.
- Melmed S (1 January 2016). Williams Textbook of Endocrinology. Elsevier Health Sciences. pp. 760–769. ISBN 978-0-323-29738-7.
- Lenehan P (12 June 2003). Anabolic Steroids. CRC Press. pp. 108–109. ISBN 978-0-415-28029-7.
- Becker KL (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 1116, 1119, 1152, 1182–1185, 1195–1197, 2146. ISBN 978-0-7817-1750-2. Archived from the original on May 5, 2017.
- Nieschlag E, Behre HM (6 December 2012). Testosterone: Action - Deficiency - Substitution. Springer Science & Business Media. pp. 1–, 9, 298, 309–331, 349–353, 366–367. ISBN 978-3-642-72185-4.
- Krishna UR, Sheriar NR, Mandecklar A (1996). Menopause. Orient Blackswan. pp. 70–. ISBN 978-81-250-0910-8.
- Brotherton J (1976). Sex Hormone Pharmacology. Academic Press. pp. 18–19, 331, 336. ISBN 978-0-12-137250-7.
- Wells BG, DiPiro JT, Schwinghammer TL, DiPiro CV (22 August 2014). Pharmacotherapy Handbook, 9/E. McGraw-Hill Education. p. 288. ISBN 978-0-07-182129-2.
- Lauritzen C (1988). "Natürliche und Synthetische Sexualhormone – Biologische Grundlagen und Behandlungsprinzipien" [Natural and Synthetic Sexual Hormones – Biological Basis and Medical Treatment Principles]. In Schneider HP, Lauritzen C, Nieschlag E (eds.). Grundlagen und Klinik der Menschlichen Fortpflanzung [Foundations and Clinic of Human Reproduction] (in German). Walter de Gruyter. pp. 229–306. ISBN 978-3110109689. OCLC 35483492.
- Apotekens informationsavdelning (1964). Apotekens synonymregister över farmacevtiska specialiteter. Apotekens informationsavdelning.
Rektandron FERRING supp. 40 mg • individ. dos. • 10 st.
- J. Bain; Wolf-Bernhard Schill; L. Schwarzstein (6 December 2012). Treatment of Male Infertility. Springer Science & Business Media. pp. 176–177. ISBN 978-3-642-68223-0.
- Snyder, P J (1984). "Clinical Use of Androgens". Annual Review of Medicine. 35 (1): 207–217. doi:10.1146/annurev.me.35.020184.001231. ISSN 0066-4219. PMID 6372655.
- Täuber, U.; Schröder, K.; Düsterberg, B.; Matthes, H. (1986). "Absolute bioavailability of testosterone after oral administration of testosterone-undecanoate and testosterone". European Journal of Drug Metabolism and Pharmacokinetics. 11 (2): 145–149. doi:10.1007/BF03189840. ISSN 0378-7966. PMID 3770015. S2CID 32305408.
- Johnsen, SvendG.; Bennett, EdgarP.; Jensen, V.Gaunø (1974). "Therapeutic effectiveness of oral testosterone". The Lancet. 304 (7895): 1473–1475. doi:10.1016/S0140-6736(74)90216-5. ISSN 0140-6736. PMID 4140393.
- Corona G, Rastrelli G, Vignozzi L, Maggi M (2012). "Emerging medication for the treatment of male hypogonadism". Expert Opin Emerg Drugs. 17 (2): 239–59. doi:10.1517/14728214.2012.683411. PMID 22612692. S2CID 22068249.
- Lemke TL, Williams DA (24 January 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 1360–. ISBN 978-1-60913-345-0.
- Nieschlag E, Behre HM, Nieschlag S (January 13, 2010). Andrology: Male Reproductive Health and Dysfunction. Springer Science & Business Media. pp. 49–54, 441–446. ISBN 978-3-540-78355-8. Archived from the original on June 23, 2016.
- Behre HM, Abshagen K, Oettel M, Hübler D, Nieschlag E (1999). "Intramuscular injection of testosterone undecanoate for the treatment of male hypogonadism: phase I studies". Eur. J. Endocrinol. 140 (5): 414–9. CiteSeerX 10.1.1.503.1752. doi:10.1530/eje.0.1400414. PMID 10229906. S2CID 22597244.
- William Llewellyn (2011). Anabolics. Molecular Nutrition Llc. pp. 212–216, 314–322. ISBN 978-0-9828280-1-4.
- Hohl A (30 March 2017). Testosterone: From Basic to Clinical Aspects. Springer. pp. 13–. ISBN 978-3-319-46086-4.
- Bagchus WM, Hust R, Maris F, Schnabel PG, Houwing NS (March 2003). "Important effect of food on the bioavailability of oral testosterone undecanoate". Pharmacotherapy. 23 (3): 319–25. doi:10.1592/phco.23.3.319.32104. PMID 12627930. S2CID 24440953.
- Schnabel PG, Bagchus W, Lass H, Thomsen T, Geurts TB (April 2007). "The effect of food composition on serum testosterone levels after oral administration of Andriol Testocaps". Clin. Endocrinol. (Oxf). 66 (4): 579–85. doi:10.1111/j.1365-2265.2007.02781.x. PMC 1859980. PMID 17371478.
- Byrne, M.M.; Nieschlag, E. (2017). "Androgens: Pharmacological Use and Abuse ☆". Reference Module in Neuroscience and Biobehavioral Psychology. doi:10.1016/B978-0-12-809324-5.03356-3. ISBN 9780128093245.
- Touitou E, Barry BW (27 November 2006). Enhancement in Drug Delivery. CRC Press. pp. 122–. ISBN 978-1-4200-0481-6.
- Lobo, Rogerio A. (2001). "Androgens in Postmenopausal Women: Production, Possible Role, and Replacement Options". Obstetric and Gynecological Survey. 56 (6): 361–376. doi:10.1097/00006254-200106000-00022. ISSN 0029-7828. PMID 11466487. S2CID 9872335.
- Buckler, H. M.; Robertson, W. R.; Wu, F. C. W. (1998). "Which Androgen Replacement Therapy for Women?1". The Journal of Clinical Endocrinology & Metabolism. 83 (11): 3920–3924. doi:10.1210/jcem.83.11.5280. ISSN 0021-972X. PMID 9814469.
- Nieschlag, Eberhard; Nieschlag, Susan (2019). "ENDOCRINE HISTORY: The history of discovery, synthesis and development of testosterone for clinical use". European Journal of Endocrinology. 180 (6): R201–R212. doi:10.1530/EJE-19-0071. ISSN 0804-4643. PMID 30959485.
- Wang, C; Eyre, D R; Clark, R; Kleinberg, D; Newman, C; Iranmanesh, A; Veldhuis, J; Dudley, R E; Berman, N; Davidson, T; Barstow, T J; Sinow, R; Alexander, G; Swerdloff, R S (1996). "Sublingual testosterone replacement improves muscle mass and strength, decreases bone resorption, and increases bone formation markers in hypogonadal men--a clinical research center study". The Journal of Clinical Endocrinology & Metabolism. 81 (10): 3654–3662. doi:10.1210/jcem.81.10.8855818. ISSN 0021-972X. PMID 8855818.
- Escamilla RF, Gordan GS (March 1951). "Sublingual administration of testosterone compounds in male hypogonadism". Ciba Clin Symp. 3 (2): 49–56. PMID 14822123.
- Brotherton J (1976). Sex Hormone Pharmacology. Academic Press. pp. 18–19, 331, 336. ISBN 978-0-12-137250-7.
- Davison, Sonia; Thipphawong, John; Blanchard, Jim; Liu, Kui; Morishige, Richard; Gonda, Igor; Okikawa, Jerry; Adams, Jennifer; Evans, Allan; Otulana, Babatunde; Davis, Susan (2005). "Pharmacokinetics and Acute Safety of Inhaled Testosterone in Postmenopausal Women". The Journal of Clinical Pharmacology. 45 (2): 177–184. doi:10.1177/0091270004269840. ISSN 0091-2700. PMID 15647410. S2CID 25919373.
- Jones H (25 September 2008). Testosterone Deficiency in Men. OUP Oxford. pp. 89–. ISBN 978-0-19-954513-1.
- Rastrelli, G.; Reisman, Y.; Ferri, S.; Prontera, O.; Sforza, A.; Maggi, M.; Corona, G. (2019). "Testosterone Replacement Therapy". Sexual Medicine. pp. 79–93. doi:10.1007/978-981-13-1226-7_8. ISBN 978-981-13-1225-0. S2CID 240176927.
- Henzl MR, Loomba PK (July 2003). "Transdermal delivery of sex steroids for hormone replacement therapy and contraception. A review of principles and practice". J Reprod Med. 48 (7): 525–40. PMID 12953327.
- Berth-Jones, John (2016). "Principles of Topical Therapy". Rook's Textbook of Dermatology. pp. 1–51. doi:10.1002/9781118441213.rtd0018. ISBN 9781118441213.
- Benedetti, Margherita Strolin; Whomsley, Rhys; Poggesi, Italo; Cawello, Willi; Mathy, François-Xavier; Delporte, Marie-Laure; Papeleu, Peggy; Watelet, Jean-Baptiste (2009). "Drug metabolism and pharmacokinetics". Drug Metabolism Reviews. 41 (3): 344–390. doi:10.1080/10837450902891295. ISSN 0360-2532. PMC 3086155. PMID 19601718.
- Ronald C. Wester; Howard I. Maibach (2 January 2002). "Regional Variation in Percutaneous Absorption". In Robert L. Bronaugh; Howard I. Maibach (eds.). Topical Absorption of Dermatological Products. CRC Press. pp. 33–42. doi:10.3109/9780203904015-6. ISBN 978-0-203-90401-5.
- Feldmann RJ, Maibach HI (February 1967). "Regional variation in percutaneous penetration of 14C cortisol in man". J. Invest. Dermatol. 48 (2): 181–3. doi:10.1038/jid.1967.29. PMID 6020682.
- Kühnert, B; Byrne, M; Simoni, M; Köpcke, W; Gerss, J; Lemmnitz, G; Nieschlag, E (2005). "Testosterone substitution with a new transdermal, hydroalcoholic gel applied to scrotal or non-scrotal skin: a multicentre trial". European Journal of Endocrinology. 153 (2): 317–326. doi:10.1530/eje.1.01964. ISSN 0804-4643. PMID 16061839.
- Iyer, R.; Mok, S. F.; Savkovic, S.; Turner, L.; Fraser, G.; Desai, R.; Jayadev, V.; Conway, A. J.; Handelsman, D. J. (2017). "Pharmacokinetics of testosterone cream applied to scrotal skin". Andrology. 5 (4): 725–731. doi:10.1111/andr.12357. ISSN 2047-2919. PMID 28334510.
- Amano, Toshiyasu; Iwamoto, Teruaki; Sato, Yoshikazu; Imao, Tetsuya; Earle, Carolyn (2018). "The efficacy and safety of short-acting testosterone ointment (Glowmin) for late-onset hypogonadism in accordance with testosterone circadian rhythm". The Aging Male. 21 (3): 170–175. doi:10.1080/13685538.2018.1471129. ISSN 1368-5538. PMID 29734846. S2CID 13701612.
- Needham S, Needham S (2018). "Case Study: Absorption of Testosterone Cream via Scrotal Delivery". Int J Pharm Compd. 22 (6): 466–468. PMID 30384346.
- Nieschlag, Eberhard (2015). "Current topics in testosterone replacement of hypogonadal men". Best Practice & Research Clinical Endocrinology & Metabolism. 29 (1): 77–90. doi:10.1016/j.beem.2014.09.008. ISSN 1521-690X. PMID 25617174.
- Potts RO, Lobo RA (May 2005). "Transdermal drug delivery: clinical considerations for the obstetrician-gynecologist". Obstet Gynecol. 105 (5 Pt 1): 953–61. doi:10.1097/01.AOG.0000161958.70059.db. PMID 15863530. S2CID 23411589.
- Davis, Susan R.; Nieschlag, Eberhard; Behre, Hermann M.; Nieschlag, Susan (26 July 2012). "Testosterone use in women". In Eberhard Nieschlag; Hermann M. Behre; Susan Nieschlag (eds.). Testosterone: Action, Deficiency, Substitution. Cambridge University Press. pp. 494–516. doi:10.1017/CBO9781139003353.024. ISBN 978-1-107-01290-5.
- Maia, Hugo; Casoy, Julio; Valente, Jorge (2009). "Testosterone replacement therapy in the climacteric: Benefits beyond sexuality". Gynecological Endocrinology. 25 (1): 12–20. doi:10.1080/09513590802360744. ISSN 0951-3590. PMID 19165658. S2CID 40325624.
- Patrick J. Culligan; Roger P. Goldberg (6 March 2007). Urogynecology in Primary Care. Springer Science & Business Media. pp. 116–. ISBN 978-1-84628-167-9.
Topical vaginal testosterone is often used in premenopausal women as a first step in the treatment of sexual dysfunction and vaginal lichen planus. Topical testosteorne preparations can be compounded in 1% to 2% formulations and should be applied up to 3 times per week.
- Maxine A. Papadakis; Stephen J. McPhee; Michael W. Rabow (11 September 2017). Current Medical Diagnosis and Treatment 2018, 57th Edition. McGraw-Hill Education. pp. 1217–1218. ISBN 978-1-259-86149-9.
Testosterone can also be compounded as a cream containing 1 mg/mL, with 1 mL applied to the abdomen daily. Vaginal testosterone is an option for postmenopausal women who cannot use systemic or vaginal estrogen due to breast cancer. Testosterone 150–300 mcg/day vaginally appears to reduce vaginal dryness and dyspareunia without increasing systemic estrogen levels.
- Joseph E. Pizzorno (2013). Textbook of Natural Medicine. Elsevier Health Sciences. pp. 1602–. ISBN 978-1-4377-2333-5.
At present, bioidentical testosterone can be obtained only from a compounding pharmacy, where 4 to 6 mg of bioidentical testosterone is generally formulated alone or together with the biestrogen or triestrogen formulation. Testosterone cream applied to the genital region can be used as an alternative delivery method. Common prescriptions are anywhere from 1 to 10 mg/g of cream.
- Morley, J. E.; Perry, H. M. (2003). "Androgens and Women at the Menopause and Beyond". The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 58 (5): M409–M416. doi:10.1093/gerona/58.5.M409. ISSN 1079-5006. PMID 12730248.
- Rogerio A. Lobo; Jennifer Kelsey; Robert Marcus (22 May 2000). Menopause: Biology and Pathobiology. Academic Press. pp. 455–. ISBN 978-0-08-053620-0.
- Hamburger C, Renaud B, Buda M, Lewis BD, Pujol JF (August 1958). "Testosterone treatment and 17-ketosteroid excretion. V. Administration of testosterone per rectum". Acta Endocrinol. 28 (4): 529–36. doi:10.1530/acta.0.0280529. PMID 13570882.
- Aakvaag A, Vogt JH (March 1969). "Plasma testosterone values in different forms of testosterone treatment". Acta Endocrinol. 60 (3): 537–42. doi:10.1530/acta.0.0600537. PMID 5395873.
- Nieschlag, E.; Cüppers, H.J.; Wiegelmann, W.; Wickings, E.J. (1976). "Bioavailability and LH-Suppressing Effect of Different Testosterone Preparations in Normal and Hypogonadal Men". Hormone Research. 7 (3): 138–145. doi:10.1159/000178721. ISSN 1423-0046. PMID 1002121.
- Lentini S, Fortunio G (1952). "Assorbimento e azione del testosterone somministrato per via rettale" [Absorption and action of testosterone administered rectally]. Clin Nuova Rass Prog Med Int (in Italian). 14 (1–2): 5–16. PMID 14945075.
- Hamburger C (February 1974). "Testosteronesuppositorier DA" [Letter: Testosterone suppositories DAK]. Ugeskr Laeger (in Danish). 136 (6): 307–8. PMID 4820554.
- Conceptual Pharmacology. Universities Press. 2010. pp. 8–. ISBN 978-81-7371-679-9.
- Yeung SJ, Escalante CP, Gagel RF (2009). Medical Care of Cancer Patients. PMPH-USA. pp. 247–. ISBN 978-1-60795-008-0.
- Morgentaler A, Traish AM (February 2009). "Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth". Eur. Urol. 55 (2): 310–20. doi:10.1016/j.eururo.2008.09.024. PMID 18838208.
- Cooper CS, Perry PJ, Sparks AE, MacIndoe JH, Yates WR, Williams RD (February 1998). "Effect of exogenous testosterone on prostate volume, serum and semen prostate specific antigen levels in healthy young men". J. Urol. 159 (2): 441–3. doi:10.1016/s0022-5347(01)63944-2. PMID 9649259.
- Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J, Bunnell TJ, Tricker R, Shirazi A, Casaburi R (July 1996). "The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men". N. Engl. J. Med. 335 (1): 1–7. doi:10.1056/NEJM199607043350101. PMID 8637535. S2CID 73721690.
- Hamburger, Christian (1952). "17-Ketosteroid Excretion and Modes of Administering Testosterone Preparations". Ciba Foundation Symposium - Steroid Hormone Administration (Book II of Colloquia on Endocrinology, Vol. 3). Novartis Foundation Symposia. pp. 304–322. doi:10.1002/9780470715154.ch7. ISBN 9780470715154. ISSN 1935-4657.
- Al-Futaisi AM, Al-Zakwani IS, Almahrezi AM, Morris D (December 2006). "Subcutaneous administration of testosterone. A pilot study report". Saudi Med J. 27 (12): 1843–6. PMID 17143361.
- Deutsch MB, Bhakri V, Kubicek K (2015). "Effects of cross-sex hormone treatment on transgender women and men". Obstet Gynecol. 125 (3): 605–10. doi:10.1097/AOG.0000000000000692. PMC 4442681. PMID 25730222.
- Olson J, Schrager SM, Clark LF, Dunlap SL, Belzer M (September 2014). "Subcutaneous Testosterone: An Effective Delivery Mechanism for Masculinizing Young Transgender Men". LGBT Health. 1 (3): 165–7. doi:10.1089/lgbt.2014.0018. PMID 26789709.
- Spratt DI, Stewart II, Savage C, Craig W, Spack NP, Chandler DW, Spratt LV, Eimicke T, Olshan JS (July 2017). "Subcutaneous Injection of Testosterone Is an Effective and Preferred Alternative to Intramuscular Injection: Demonstration in Female-to-Male Transgender Patients". The Journal of Clinical Endocrinology & Metabolism. 102 (7): 2349–2355. doi:10.1210/jc.2017-00359. PMID 28379417.
- McFarland J, Craig W, Clarke NJ, Spratt DI (August 2017). "Serum Testosterone Concentrations Remain Stable Between Injections in Patients Receiving Subcutaneous Testosterone". Journal of the Endocrine Society. 1 (8): 1095–1103. doi:10.1210/js.2017-00148. PMC 5686655. PMID 29264562.
- Wilson DM, Kiang TK, Ensom MH (March 2018). "Pharmacokinetics, safety, and patient acceptability of subcutaneous versus intramuscular testosterone injection for gender-affirming therapy: A pilot study". Am J Health Syst Pharm. 75 (6): 351–358. doi:10.2146/ajhp170160. PMID 29367424. S2CID 3886536.
- Singh GK, Turner L, Desai R, Jimenez M, Handelsman DJ (July 2014). "Pharmacokinetic-pharmacodynamic study of subcutaneous injection of depot nandrolone decanoate using dried blood spots sampling coupled with ultrapressure liquid chromatography tandem mass spectrometry assays". The Journal of Clinical Endocrinology & Metabolism. 99 (7): 2592–8. doi:10.1210/jc.2014-1243. PMID 24684468.
- Bhasin S (13 February 1996). Pharmacology, Biology, and Clinical Applications of Androgens: Current Status and Future Prospects. John Wiley & Sons. pp. 462–. ISBN 978-0-471-13320-9.
- Kumar P, Clark ML (4 June 2012). Kumar and Clark's Clinical Medicine. Elsevier Health Sciences. pp. 976–. ISBN 978-0-7020-5304-7.
- Karch SB (21 December 2006). Drug Abuse Handbook, Second Edition. CRC Press. pp. 700–. ISBN 978-1-4200-0346-8.
- Wecker L, Watts S, Faingold C, Dunaway G, Crespo L (1 April 2009). Brody's Human Pharmacology. Elsevier Health Sciences. pp. 468–469. ISBN 978-0-323-07575-6.
- Becker KL (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 1116, 1119, 1183. ISBN 978-0-7817-1750-2. Archived from the original on June 28, 2014.
- Thieme D, Hemmersbach P (18 December 2009). Doping in Sports. Springer Science & Business Media. pp. 53–. ISBN 978-3-540-79088-4.
- Karch SB, Drummer O (26 December 2001). Karch's Pathology of Drug Abuse (third ed.). CRC Press. pp. 486–. ISBN 978-1-4200-4211-5.
- A. Labhart (6 December 2012). Clinical Endocrinology: Theory and Practice. Springer Science & Business Media. pp. 450–. ISBN 978-3-642-96158-8.
Further reading
- Behre, Hermann M.; Nieschlag, Eberhard; Nieschlag, Eberhard; Behre, Hermann M.; Nieschlag, Susan (26 July 2012). "Testosterone preparations for clinical use in males". In Eberhard Nieschlag; Hermann M. Behre; Susan Nieschlag (eds.). Testosterone: Action, Deficiency, Substitution. Cambridge University Press. pp. 309–335. doi:10.1017/CBO9781139003353.016. ISBN 978-1-107-01290-5.
- Byrne, M.M.; Nieschlag, E. (2017). "Androgens: Pharmacological Use and Abuse". Reference Module in Neuroscience and Biobehavioral Psychology. doi:10.1016/B978-0-12-809324-5.03356-3.