CYP2R1
CYP2R1 is cytochrome P450 2R1, an enzyme which is the principal vitamin D 25-hydroxylase.[5][6] In humans it is encoded by the CYP2R1 gene located on chromosome 11p15.2.[7] It is expressed in the endoplasmic reticulum in liver, where it performs the first step in the activation of vitamin D by catalyzing the formation of 25-hydroxyvitamin D.[8]
Vitamin D 25-hydroxylase activity is also possessed by some other cytochrome P450 enzymes, in particular CYP27A1, which is found in mitochondria.[8][9]
Function
.png.webp)
CYP2R1 is a member of the cytochrome P450 superfamily of enzymes.[10] The cytochrome P450 proteins are mono-oxygenases which catalyze many reactions involved in drug metabolism and synthesis of cholesterol, steroids and other lipids.[10]
CYP2R1 is present in the endoplasmic reticulum of the liver (the microsomal fraction). It has 25-hydroxylase activity, which converts cholecalciferol (vitamin D3) into calcifediol (25-hydroxyvitamin D3, also known as calcidiol), the major circulatory form of the vitamin.[8][9] CYP2R1 will also hydroxylate ergocalciferol (vitamin D2), derived from dietary sources, into 25-hydroxyvitamin D2 (ercalcidiol).[8] These 25-hydroxylated forms of vitamin D, together known as 25(OH)D, bind strongly to the vitamin D-binding protein in blood and are the principal circulating forms of vitamin D. These are commonly measured to determine a person's vitamin D status and establish vitamin D deficiency.[11]
Calcifediol is subsequently converted by the action of 25-hydroxyvitamin D3 1-alpha-hydroxylase to calcitriol, the active form of vitamin D3 which binds to the vitamin D receptor (VDR) and mediates most of the physiological hormonal actions of vitamin D.[5]
Clinical significance
The conversion of vitamin D, especially cholecalciferol, to 25(OH)D (calcifediol) is one of the key steps in the vitamin D hormonal system. The CYP2R1 enzymatic activity achieving this process was previously thought to be constitutively expressed and stable, so that serum 25(OH)D was a measure of the supply of vitamin D.[9]
CYP2R1 is now known to be regulated, with variations in the expression and activity of CYP2R1 affecting circulating 25(OH)D.[9] Low levels of CYP2R1 activity have been found after 24 hour fasting, in obesity, type 1 and type 2 diabetes[12] and are decreased by glucocorticoids such as dexamethasone.[9] These conditions are known to be linked to low blood levels of 25(OH)D, where even large doses of vitamin D may not produce an improvement, which can be explained by enzyme activities being low.[9]
Polymorphic variations in CYP2R1
Polymorphic variations in the CYP2R1 gene have the greatest effect on individual serum 25(OH)D concentrations compared with other gene variations.[13] An inherited mutation in the CYP2R1 gene L99P, which results in the substitution of a proline for a leucine residue at codon 99, eliminates the enzyme activity and is associated with vitamin D-dependent rickets type IB. Another variant is K242N, where lysine at position 242 is substituted by asparagine, give a similar phenotype.[14] Symptoms are low circulating levels of 25(OH)D and classic symptoms of vitamin D deficiency.[5][15]
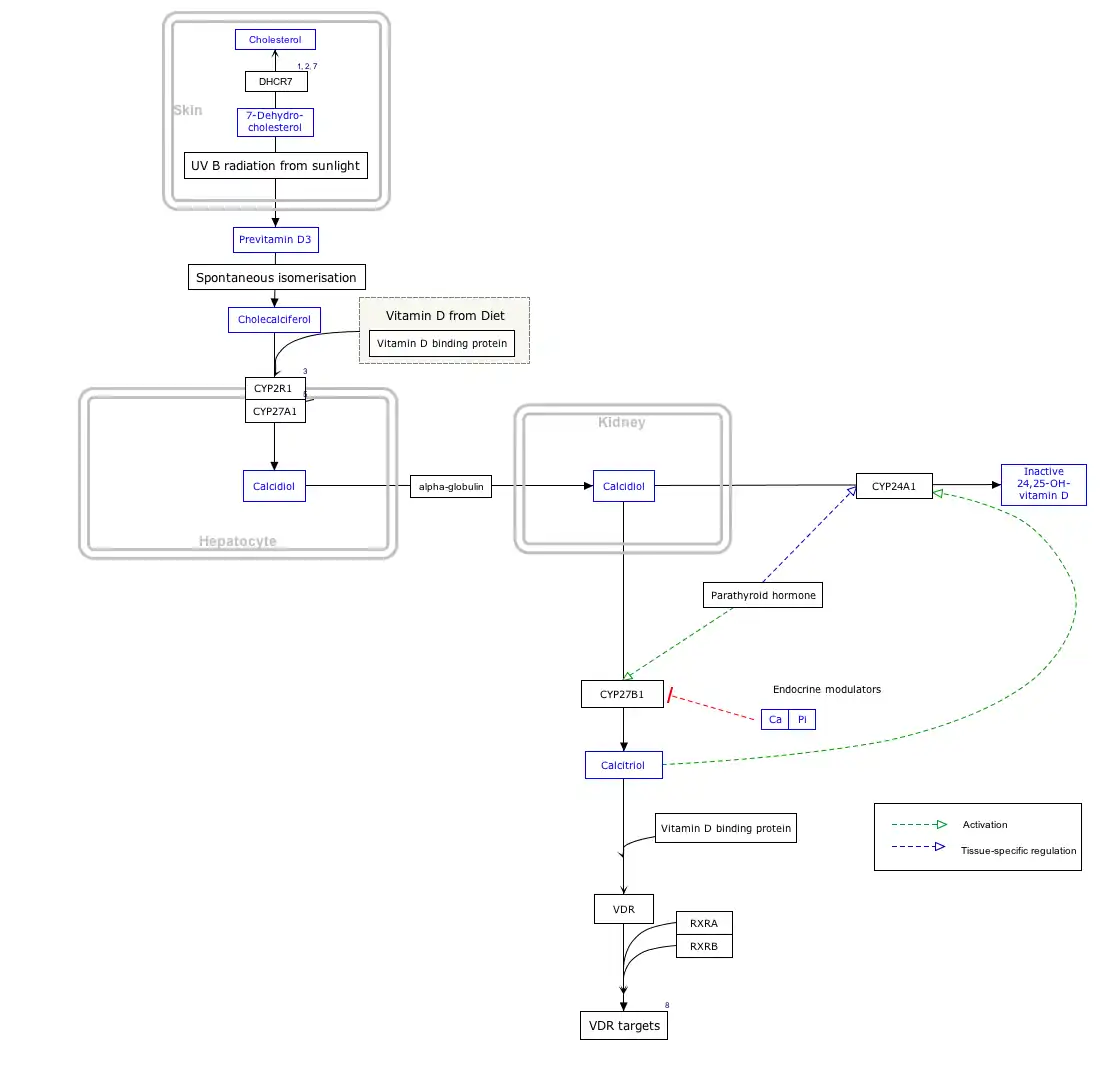
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective articles. [§ 1]
- The interactive pathway map can be edited at WikiPathways: "VitaminDSynthesis_WP1531".
Studies in mice
Model organisms have been used in the study of CYP2R1 function. Mice have been generated with knockout of Cyp2r1 and both Cyp2r1 and Cyp27a1.[16] A conditional knockout mouse line called Cyp2r1tm1b(EUCOMM)Wtsi has been generated and animals have undergone a standardized phenotypic screen.[17][18]
References
- GRCh38: Ensembl release 89: ENSG00000186104 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000030670 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Cheng JB, Motola DL, Mangelsdorf DJ, Russell DW (September 2003). "De-orphanization of cytochrome P450 2R1: a microsomal vitamin D 25-hydroxilase". J Biol Chem. 278 (39): 38084–93. doi:10.1074/jbc.M307028200. PMC 4450819. PMID 12867411.
- Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, Russell DW (May 2004). "Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase". Proc Natl Acad Sci U S A. 101 (20): 7711–5. Bibcode:2004PNAS..101.7711C. doi:10.1073/pnas.0402490101. PMC 419671. PMID 15128933.
- "Entrez Gene: CYP2R1 cytochrome P450, family 2, subfamily R, polypeptide 1".
- Bikle DD (March 2014). "Vitamin D metabolism, mechanism of action, and clinical applications". Chemistry & Biology. 21 (3): 319–29. doi:10.1016/j.chembiol.2013.12.016. PMC 3968073. PMID 24529992.
- Bouillon R, Bikle D (November 2019). "Vitamin D Metabolism Revised: Fall of Dogmas". Journal of Bone and Mineral Research (Review). 34 (11): 1985–1992. doi:10.1002/jbmr.3884. PMC 9000993. PMID 31589774.
- Nelson DR (Dec 2002). "Comparison of P450s from human and fugu: 420 million years of vertebrate P450 evolution". Arch Biochem Biophys. 409 (1): 18–24. doi:10.1016/S0003-9861(02)00553-2. PMID 12464240.
- "Office of Dietary Supplements - Vitamin D". ods.od.nih.gov. 9 October 2020. Retrieved 7 March 2021.
- Ramos-Lopez E, Brück P, Jansen T, et al. (2008). "CYP2R1 (vitamin D 25-hydroxylase) gene is associated with susceptibility to type 1 diabetes and vitamin D levels in Germans". Diabetes Metab. Res. Rev. 23 (8): 631–6. doi:10.1002/dmrr.719. PMID 17607662. S2CID 376070.
- Manousaki D, Dudding T, Haworth S, Hsu YH, Liu CT, Medina-Gómez C, et al. (December 2018). "Low-Frequency Synonymous Coding Variation in CYP2R1 Has Large Effects on Vitamin D Levels and Risk of Multiple Sclerosis". American Journal of Human Genetics. 103 (6): 1053. doi:10.1016/j.ajhg.2018.11.010. PMC 6288274. PMID 30526863.
- Thacher TD, Levine MA (October 2017). "CYP2R1 mutations causing vitamin D-deficiency rickets". J Steroid Biochem Mol Biol. 173: 333–336. doi:10.1016/j.jsbmb.2016.07.014. PMID 27473561. S2CID 1693344.
- Molin A, Wiedemann A, Demers N, Kaufmann M, Do Cao J, Mainard L, et al. (September 2017). "Vitamin D-Dependent Rickets Type 1B (25-Hydroxylase Deficiency): A Rare Condition or a Misdiagnosed Condition?". Journal of Bone and Mineral Research. 32 (9): 1893–1899. doi:10.1002/jbmr.3181. PMID 28548312.
- Zhu JG, Ochalek JT, Kaufmann M, Jones G, Deluca HF (September 2013). "CYP2R1 is a major, but not exclusive, contributor to 25-hydroxyvitamin D production in vivo". Proc Natl Acad Sci U S A. 110 (39): 15650–5. Bibcode:2013PNAS..11015650Z. doi:10.1073/pnas.1315006110. PMC 3785760. PMID 24019477.
- "Cyp2r1 Mouse Gene Details". www.mousephenotype.org. International Mouse Phenotyping Consortium. Retrieved 8 March 2021.
- Skarnes WC, Rosen B, West AP, Koutsourakis M, Bushell W, Iyer V, et al. (Jun 2011). "A conditional knockout resource for the genome-wide study of mouse gene function". Nature. 474 (7351): 337–42. doi:10.1038/nature10163. PMC 3572410. PMID 21677750.
External links
- Human CYP2R1 genome location and CYP2R1 gene details page in the UCSC Genome Browser.