CYP3A5
Cytochrome P450 3A5 is a protein that in humans is encoded by the CYP3A5 gene.
Tissue distribution
CYP3A5 encodes a member of the cytochrome P450 superfamily of enzymes. Like most of the cytochrome P450, the CYP3A5 is expressed in the prostate and the liver.[5] It is also expressed in epithelium of the small intestine and large intestine for uptake and in small amounts in the bile duct, nasal mucosa, kidney, adrenal cortex, epithelium of the gastric mucosa with intestinal metaplasia, gallbladder, intercalated ducts of the pancreas, chief cells of the parathyroid and the corpus luteum of the ovary (at protein level).[5]
Clinical significance
The cytochrome P450 proteins are monooxygenases which catalyze many reactions involved in drug metabolism and synthesis of cholesterol, steroids and other lipids. This protein localizes to the endoplasmic reticulum and its expression is induced by glucocorticoids and some pharmacological agents. The enzyme metabolizes drugs such as nifedipine and cyclosporine as well as the steroid hormones testosterone, progesterone and androstenedione. This gene is part of a cluster of cytochrome P450 genes on chromosome 7q21.1. This cluster includes a pseudogene, CYP3A5P1, which is very similar to CYP3A5. This similarity has caused some difficulty in determining whether cloned sequences represent the gene or the pseudogene.[6]
CYP3A4/3A5 are a group of heme-thiolate monooxygenases. In liver microsomes, this enzyme is involved in an NADPH-dependent electron transport pathway. It oxidizes a variety of structurally unrelated compounds, including steroids, fatty acids, and xenobiotics.[5] Immunoblot analysis of liver microsomes showed that CYP3A5 is expressed as a 52.5-kD protein, whereas CYP3A4 migrates as a 52.0-kD protein.[7] The human CYP3A subfamily, CYP3A4, CYP3A5, CYP3A7 and CYP3A43, is one of the most versatile of the biotransformation systems that facilitate the elimination of drugs (37% of the 200 most frequently prescribed drugs in the U.S.[8]).
CYP3A4 and CYP3A5 together account for approximately 30% of hepatic cytochrome P450, and approximately half of medications that are oxidatively metabolized by P450 are CYP3A substrates.[9] Both CYP3A4 and CYP3A5 are expressed in liver and intestine, with CYP3A5 being the predominant form expressed in extrahepatic tissues.[9]
Selective inhibition and therapeutic relevance
The (wild-type) CYP3A enzymes have traditionally been thought of as functionally redundant, distinguishable mostly by expression patterns. Since CYP3A5 is almost always expressed at significantly lower levels than CYP3A4, an understanding of its clinical significance was limited. Most studies suggesting any non-overlapping metabolic functions apart from CYP3A4 were limited to small differences in metabolites produced from drugs which themselves were still substrates of CYP3A4.[10] However, in 2016 it was found that CYP3A5 mediated acquired drug resistance in pancreatic ductal adenocarcinoma, a type of pancreatic cancer.[11] This not only showed a context of selective CYP3A5 expression, but also demonstrated a therapeutic need for selective CYP3A5 inhibition and hinted that its metabolic role was not completely redundant with CYP3A4. Indeed, chemical tools would soon after be developed which could demonstrate and probe the selective CYP3A5 metabolic activity.
In 2020, Wright et al. reported the first CYP3A5-selective inhibitor clobetasol propionate.[12] The study demonstrated a strong inhibition of CYP3A5 and showed its high selectivity over other CYP3A enzymes including CYP3A4. It was proposed that clobetasol propionate differentially occupied the binding site of CYP3A5 compared to CYP3A4 (which would later become validated with subsequent studies).[13]
Allele distribution
The CYP3A5 gene has several functional variants, which vary depending on ethnicity. The CYP3A5*1 allele is associated with a normal metabolization of medication. It is most common among individuals native to Sub-Equatorial Africa, though the mutation also occurs at low frequencies in other populations. The CYP3A5*3 allele is linked with a poor metabolization of medication. It is near fixation in Europe, and is likewise found at high frequencies in West Asia and Central Asia, as well as among Afro-Asiatic (Hamitic-Semitic) speaking populations in North Africa and the Horn of Africa. Additionally, the mutation occurs at moderate-to-high frequencies in South Asia, Southeast Asia and East Asia, and at low frequencies in Sub-Equatorial Africa.[14][15]
Global distribution of the CYP3A5 alleles:[15]
| Population | CYP3A5*1 | CYP3A5*3 | CYP3A5*6 | CYP3A5*7 |
|---|---|---|---|---|
| Adygei | 12% | 88% | ||
| Afar | 35% | 65% | 18% | 0% |
| African Americans | 63% | 37% | 12% | 21% |
| Algerians (North) | 19% | 81% | 5% | 1% |
| Amhara | 33% | 67% | 15% | 0% |
| Anatolian Turks | 9% | 91% | 0% | 0% |
| Armenians (South) | 5% | 95% | 0% | 0% |
| Asante | 89% | 11% | 22% | 7% |
| Ashkenazi Jews | 3% | 97% | 0% | 0% |
| Balochi | 20% | 80% | ||
| Bantu (Kenya) | 83% | 17% | ||
| Bantu (South Africa) | 74% | 26% | 18% | 10% |
| Bantu (Uganda) | 96% | 4% | 22% | 21% |
| Basques (French) | 4% | 96% | ||
| Bedouin (Israel) | 17% | 83% | ||
| Berbers (Morocco) | 20% | 80% | 4% | 1% |
| Biaka Pygmies | 89% | 11% | ||
| Brahui | 12% | 88% | ||
| Britons (England and Scotland) | 35% | 65% | 0% | |
| Bulsa | 81% | 19% | 16% | 13% |
| Burusho | 22% | 78% | ||
| Cameroonian (Lake Chad) | 76% | 24% | 32% | 7% |
| Canadian Caucasians | 7% | 93% | 0% | 0% |
| Chagga | 74% | 26% | 14% | 9% |
| Chewa | 85% | 15% | 16% | 17% |
| Chinese | 25% | 75% | 0% | |
| Chinese (Denver, Colorado) | 25% | 75% | ||
| Colombians | 15% | 85% | ||
| Colombians (Medellian) | 48% | 52% | 2% | |
| Congolese (Brazzaville) | 80% | 20% | 12% | 9% |
| Dai | 45% | 55% | ||
| Druze | 8% | 92% | ||
| Daur | 15% | 85% | ||
| East Asian | 31% | 69% | 0% | 0% |
| European | 2% | 98% | 0% | 0% |
| Finns | 45% | 55% | 0% | |
| French | 8%-9% | 91%-92% | 0% | 0% |
| Gabonese | 79% | 21% | 19% | 19% |
| Gambians | 79% | 21% | 20% | 12% |
| Germans | 7% | 93% | ||
| Gujarati (Houston, Texas) | 25% | 75% | ||
| Han | 25% | 75% | ||
| Han (Beijing) | 28% | 72% | 0% | |
| Han (Southern) | 47% | 53% | 0% | |
| Hazara | 25% | 75% | ||
| Hezhen | 15% | 85% | ||
| Hispanic | 25% | 75% | 0% | 0% |
| Iberians | 39% | 61% | 0% | |
| Igbo | 87% | 13% | 18% | 9% |
| Indians | 41% | 59% | 0% | |
| Italians (Bergamo) | 18% | 82% | ||
| Italians (Sardinia) | 5% | 95% | ||
| Italians (Tuscany) | 5%-6% | 94%-95% | 0.5% | |
| Japanese | 23% | 77% | 0% | |
| Japanese (Tokyo) | 26% | 74% | 0.004% | |
| Kalash | 24% | 76% | ||
| Karitiana | 23% | 77% | ||
| Kasena | 78% | 22% | 17% | 13% |
| Khmer | 27% | 73% | ||
| Koreans | 19% | 81% | 0% | |
| Kotoko | 73% | 27% | 23% | 5% |
| Lahu | 25% | 75% | ||
| Lemba | 87% | 13% | 25% | 15% |
| Lomwe | 83% | 17% | 22% | 11% |
| Luhya (Webuye, Kenya) | 86% | 14% | 26% | |
| Maale | 51% | 49% | 15% | 1% |
| Maasai (Kinyawa, Kenya) | 51% | 49% | 14% | |
| Makrani | 14% | 86% | ||
| Malay | 39% | 61% | 0% | |
| Malawians | 79% | 21% | 14% | 14% |
| Mandenka | 69% | 31% | ||
| Manjak | 79% | 21% | 23% | 7% |
| Maya | 29% | 71% | ||
| Mayo Darle | 73% | 27% | 25% | 6% |
| Mbuti Pygmies | 93% | 7% | ||
| Melanesians | 18% | 82% | ||
| Mestizo (El Salvador and Nicaragua) | 24% | 76% | ||
| Mestizo (Ecuador) | 12% | 88% | ||
| Mexicans (Los Angeles) | 25% | 75% | 2% | |
| Miaozu | 35% | 65% | ||
| Mongola | 35% | 65% | ||
| Mozabite | 16% | 84% | ||
| Naxi | 28% | 72% | ||
| Ngoni | 89% | 11% | 33% | 6% |
| North American Caucasians | 9% | 90% | ||
| Orogen | 10% | 90% | ||
| Orcadians | 16% | 84% | ||
| Oromo | 35% | 65% | 14% | 0% |
| Papuans | 21% | 79% | ||
| Palestinians | 18% | 82% | ||
| Pathan | 12% | 88% | ||
| Pima | 54% | 46% | ||
| Puerto Ricans | 56% | 44% | 5% | |
| Russians | 8% | 92% | ||
| San (Namibia) | 93% | 7% | ||
| Sena | 84% | 16% | 23% | 16% |
| Sephardi Jews | 11% | 89% | 0% | 0% |
| She | 45% | 55% | ||
| Shewa Arabs | 60% | 40% | 22% | 7% |
| Shona | 22% | 78% | 22% | 10% |
| Sindhi | 18% | 82% | ||
| Somie (Cameroonian Grassfields) | 77% | 23% | 18% | 10% |
| Southern Sudanese | 76% | 24% | 33% | 3% |
| Spaniard | 9% | 91% | ||
| Sudanese (Northern) | 40% | 60% | 11% | 0% |
| Sudanese (Kordofan) | 55% | 45% | 20% | 2% |
| Surui | 17% | 83% | ||
| Swedes | 7% | 93% | 0% | 0% |
| Tanzanians | 81% | 19% | 19% | 12% |
| Tu | 10% | 90% | ||
| Tujia | 35% | 65% | ||
| Tunisian | 19% | 81% | 1% | 0% |
| Uygur | 5% | 95% | ||
| Wolof | 73% | 27% | 18% | 9% |
| Xibo | 22% | 78% | ||
| Yao | 82% | 18% | 13% | 9% |
| Yakuts | 10% | 90% | ||
| Yemeni (Hadramaut) | 15% | 85% | 3% | 1% |
| Yemeni (Sena and Msila) | 42% | 58% | 12% | 3% |
| Yizu | 20% | 80% | ||
| Yoruba | 83%-94% | 6%-17% | 17%-75% | 0% |
| Zimbabweans (Mposi) | 84% | 16% | 16% | 19% |
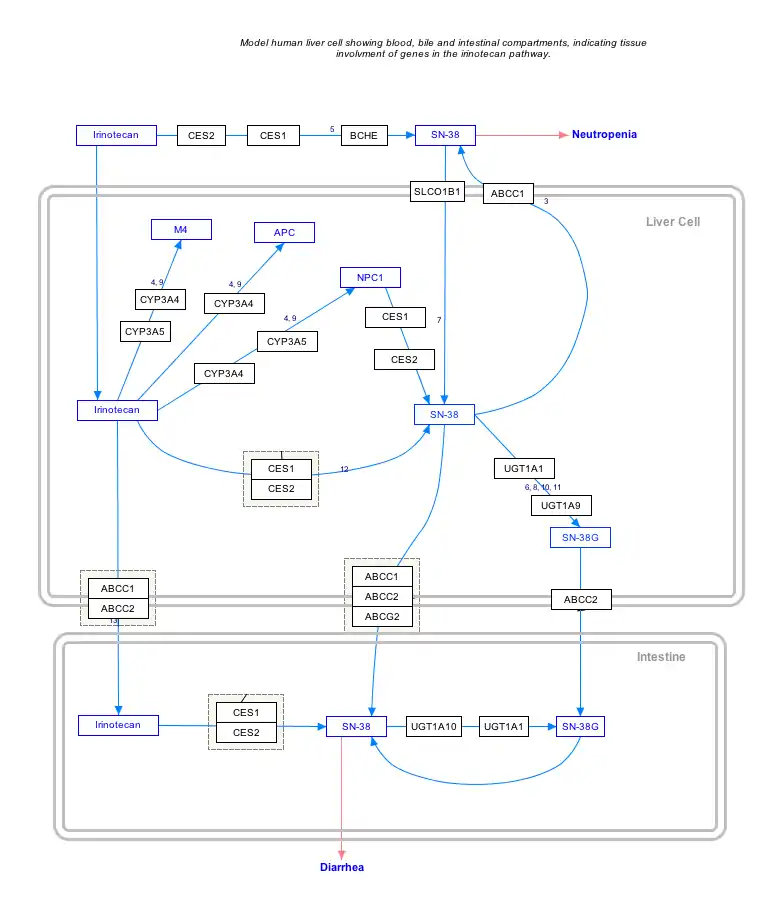
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective articles. [§ 1]
- The interactive pathway map can be edited at WikiPathways: "IrinotecanPathway_WP229".
See also
References
- GRCh38: Ensembl release 89: ENSG00000106258 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000038656 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "P08684-CP3A4_Human". UniProt. UniProt. Retrieved November 11, 2014.
- "Entrez Gene: CYP3A5 cytochrome P450, family 3, subfamily A, polypeptide 5".
- "CYTOCHROME P450, SUBFAMILY IIIA, POLYPEPTIDE 5; CYP3A5". OMIM. Retrieved November 11, 2014.
- Zanger UM, Turpeinen M, Klein K, Schwab M (2008). "Functional pharmacogenetics/genomics of human cytochromes P450 involved in drug biotransformation". Analytical and Bioanalytical Chemistry. 392 (6): 1093–108. doi:10.1007/s00216-008-2291-6. PMID 18695978. S2CID 33827704.
- "CYP3A5". PharmGKB. Retrieved November 11, 2014.
- Dennison JB, Kulanthaivel P, Barbuch RJ, Renbarger JL, Ehlhardt WJ, Hall SD (August 2006). "Selective metabolism of vincristine in vitro by CYP3A5". Drug Metabolism and Disposition. 34 (8): 1317–1327. doi:10.1124/dmd.106.009902. PMID 16679390. S2CID 1225633.
- Noll EM, Eisen C, Stenzinger A, Espinet E, Muckenhuber A, Klein C, et al. (March 2016). "CYP3A5 mediates basal and acquired therapy resistance in different subtypes of pancreatic ductal adenocarcinoma". Nature Medicine. 22 (3): 278–287. doi:10.1038/nm.4038. PMC 4780258. PMID 26855150.
- Wright WC, Chenge J, Wang J, Girvan HM, Yang L, Chai SC, et al. (February 2020). "Clobetasol Propionate Is a Heme-Mediated Selective Inhibitor of Human Cytochrome P450 3A5". Journal of Medicinal Chemistry. 63 (3): 1415–1433. doi:10.1021/acs.jmedchem.9b02067. PMC 7087482. PMID 31965799.
- Wang J, Buchman CD, Seetharaman J, Miller DJ, Huber AD, Wu J, et al. (November 2021). "Unraveling the Structural Basis of Selective Inhibition of Human Cytochrome P450 3A5". Journal of the American Chemical Society. 143 (44): 18467–18480. doi:10.1021/jacs.1c07066. PMC 8594567. PMID 34648292.
- Valente C, Alvarez L, Marks SJ, Lopez-Parra AM, Parson W, Oosthuizen O, Oosthuizen E, Amorim A, Capelli C, Arroyo-Pardo E, Gusmão L, Prata MJ (28 May 2015). "Exploring the relationship between lifestyles, diets and genetic adaptations in humans". BMC Genetics. 16 (55): 55. doi:10.1186/s12863-015-0212-1. PMC 4445807. PMID 26018448.
- Bains RK. "Molecular diversity and population structure at the CYP3A5 gene in Africa" (PDF). University College London. Retrieved 13 June 2016.
Further reading
- Smith G, Stubbins MJ, Harries LW, Wolf CR (December 1998). "Molecular genetics of the human cytochrome P450 monooxygenase superfamily". Xenobiotica. 28 (12): 1129–65. doi:10.1080/004982598238868. PMID 9890157.
- Lee SJ, Goldstein JA (June 2005). "Functionally defective or altered CYP3A4 and CYP3A5 single nucleotide polymorphisms and their detection with genotyping tests". Pharmacogenomics. 6 (4): 357–71. doi:10.1517/14622416.6.4.357. PMID 16004554.
- Aoyama T, Yamano S, Waxman DJ, Lapenson DP, Meyer UA, Fischer V, Tyndale R, Inaba T, Kalow W, Gelboin HV (June 1989). "Cytochrome P-450 hPCN3, a novel cytochrome P-450 IIIA gene product that is differentially expressed in adult human liver. cDNA and deduced amino acid sequence and distinct specificities of cDNA-expressed hPCN1 and hPCN3 for the metabolism of steroid hormones and cyclosporine". The Journal of Biological Chemistry. 264 (18): 10388–95. doi:10.1016/S0021-9258(18)81632-5. PMID 2732228.
- Schuetz JD, Molowa DT, Guzelian PS (November 1989). "Characterization of a cDNA encoding a new member of the glucocorticoid-responsive cytochromes P450 in human liver". Archives of Biochemistry and Biophysics. 274 (2): 355–65. doi:10.1016/0003-9861(89)90449-9. PMID 2802615.
- Murray GI, Pritchard S, Melvin WT, Burke MD (May 1995). "Cytochrome P450 CYP3A5 in the human anterior pituitary gland". FEBS Letters. 364 (1): 79–82. doi:10.1016/0014-5793(95)00367-I. PMID 7750548. S2CID 28711803.
- Jounaïdi Y, Guzelian PS, Maurel P, Vilarem MJ (December 1994). "Sequence of the 5'-flanking region of CYP3A5: comparative analysis with CYP3A4 and CYP3A7". Biochemical and Biophysical Research Communications. 205 (3): 1741–7. doi:10.1006/bbrc.1994.2870. PMID 7811260.
- McKinnon RA, Burgess WM, Hall PM, Roberts-Thomson SJ, Gonzalez FJ, McManus ME (February 1995). "Characterisation of CYP3A gene subfamily expression in human gastrointestinal tissues". Gut. 36 (2): 259–67. doi:10.1136/gut.36.2.259. PMC 1382414. PMID 7883227.
- Kolars JC, Lown KS, Schmiedlin-Ren P, Ghosh M, Fang C, Wrighton SA, Merion RM, Watkins PB (October 1994). "CYP3A gene expression in human gut epithelium". Pharmacogenetics. 4 (5): 247–59. doi:10.1097/00008571-199410000-00003. PMID 7894497.
- Lown KS, Kolars JC, Thummel KE, Barnett JL, Kunze KL, Wrighton SA, Watkins PB (1995). "Interpatient heterogeneity in expression of CYP3A4 and CYP3A5 in small bowel. Lack of prediction by the erythromycin breath test". Drug Metabolism and Disposition. 22 (6): 947–55. PMID 7895614.
- Schuetz JD, Beach DL, Guzelian PS (February 1994). "Selective expression of cytochrome P450 CYP3A mRNAs in embryonic and adult human liver". Pharmacogenetics. 4 (1): 11–20. doi:10.1097/00008571-199402000-00002. PMID 8004129.
- Maruyama K, Sugano S (January 1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Schuetz JD, Schuetz EG, Thottassery JV, Guzelian PS, Strom S, Sun D (January 1996). "Identification of a novel dexamethasone responsive enhancer in the human CYP3A5 gene and its activation in human and rat liver cells". Molecular Pharmacology. 49 (1): 63–72. PMID 8569713.
- Jounaïdi Y, Hyrailles V, Gervot L, Maurel P (April 1996). "Detection of CYP3A5 allelic variant: a candidate for the polymorphic expression of the protein?". Biochemical and Biophysical Research Communications. 221 (2): 466–70. doi:10.1006/bbrc.1996.0618. PMID 8619878.
- Hakkola J, Pasanen M, Hukkanen J, Pelkonen O, Mäenpää J, Edwards RJ, Boobis AR, Raunio H (February 1996). "Expression of xenobiotic-metabolizing cytochrome P450 forms in human full-term placenta". Biochemical Pharmacology. 51 (4): 403–11. doi:10.1016/0006-2952(95)02184-1. PMID 8619884.
- Hakkola J, Raunio H, Purkunen R, Pelkonen O, Saarikoski S, Cresteil T, Pasanen M (July 1996). "Detection of cytochrome P450 gene expression in human placenta in first trimester of pregnancy". Biochemical Pharmacology. 52 (2): 379–83. doi:10.1016/0006-2952(96)00216-X. PMID 8694864.
- Huang Z, Fasco MJ, Figge HL, Keyomarsi K, Kaminsky LS (August 1996). "Expression of cytochromes P450 in human breast tissue and tumors". Drug Metabolism and Disposition. 24 (8): 899–905. PMID 8869826.
- Kivistö KT, Bookjans G, Fromm MF, Griese EU, Münzel P, Kroemer HK (September 1996). "Expression of CYP3A4, CYP3A5 and CYP3A7 in human duodenal tissue". British Journal of Clinical Pharmacology. 42 (3): 387–9. doi:10.1046/j.1365-2125.1996.42615.x. PMC 2042681. PMID 8877031.
- Janardan SK, Lown KS, Schmiedlin-Ren P, Thummel KE, Watkins PB (October 1996). "Selective expression of CYP3A5 and not CYP3A4 in human blood". Pharmacogenetics. 6 (5): 379–85. doi:10.1097/00008571-199610000-00001. PMID 8946469.
- Anttila S, Hukkanen J, Hakkola J, Stjernvall T, Beaune P, Edwards RJ, Boobis AR, Pelkonen O, Raunio H (March 1997). "Expression and localization of CYP3A4 and CYP3A5 in human lung". American Journal of Respiratory Cell and Molecular Biology. 16 (3): 242–9. doi:10.1165/ajrcmb.16.3.9070608. PMID 9070608.
- Hukkanen J, Hakkola J, Anttila S, Piipari R, Karjalainen A, Pelkonen O, Raunio H (October 1997). "Detection of mRNA encoding xenobiotic-metabolizing cytochrome P450s in human bronchoalveolar macrophages and peripheral blood lymphocytes". Molecular Carcinogenesis. 20 (2): 224–30. doi:10.1002/(SICI)1098-2744(199710)20:2<224::AID-MC9>3.0.CO;2-M. PMID 9364212. S2CID 25129993.
External links
- Human CYP3A5 genome location and CYP3A5 gene details page in the UCSC Genome Browser.