Algae
| Algae An informal term for a diverse group of photosynthetic eukaryotes Temporal range: | |
|---|---|
 | |
| A variety of algae growing on the sea bed in shallow waters | |
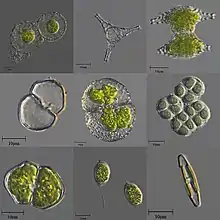 | |
| A variety of microscopic unicellular and colonial freshwater algae | |
| Scientific classification | |
| Groups included | |
| |
| Typically excluded: | |
| |
Algae (/ˈældʒiː, ˈælɡiː/; singular alga /ˈælɡə/) is an informal term for a large and diverse group of photosynthetic eukaryotic organisms. It is a polyphyletic grouping that includes species from multiple distinct clades. Included organisms range from unicellular microalgae, such as Chlorella, Prototheca and the diatoms, to multicellular forms, such as the giant kelp, a large brown alga which may grow up to 50 metres (160 ft) in length. Most are aquatic and autotrophic (they generate food internally) and lack many of the distinct cell and tissue types, such as stomata, xylem and phloem that are found in land plants. The largest and most complex marine algae are called seaweeds, while the most complex freshwater forms are the Charophyta, a division of green algae which includes, for example, Spirogyra and stoneworts.
No definition of algae is generally accepted. One definition is that algae "have chlorophyll a as their primary photosynthetic pigment and lack a sterile covering of cells around their reproductive cells".[3] Likewise, the colorless Prototheca under Chlorophyta are all devoid of any chlorophyll. Although cyanobacteria are often referred to as "blue-green algae", most authorities exclude all prokaryotes from the definition of algae.[4][5]
Algae constitute a polyphyletic group[4] since they do not include a common ancestor, and although their plastids seem to have a single origin, from cyanobacteria,[6] they were acquired in different ways. Green algae are examples of algae that have primary chloroplasts derived from endosymbiotic cyanobacteria. Diatoms and brown algae are examples of algae with secondary chloroplasts derived from an endosymbiotic red alga.[7] Algae exhibit a wide range of reproductive strategies, from simple asexual cell division to complex forms of sexual reproduction.[8]
Algae lack the various structures that characterize land plants, such as the phyllids (leaf-like structures) of bryophytes, rhizoids of nonvascular plants, and the roots, leaves, and other organs found in tracheophytes (vascular plants). Most are phototrophic, although some are mixotrophic, deriving energy both from photosynthesis and uptake of organic carbon either by osmotrophy, myzotrophy, or phagotrophy. Some unicellular species of green algae, many golden algae, euglenids, dinoflagellates, and other algae have become heterotrophs (also called colorless or apochlorotic algae), sometimes parasitic, relying entirely on external energy sources and have limited or no photosynthetic apparatus.[9][10][11] Some other heterotrophic organisms, such as the apicomplexans, are also derived from cells whose ancestors possessed plastids, but are not traditionally considered as algae. Algae have photosynthetic machinery ultimately derived from cyanobacteria that produce oxygen as a by-product of photosynthesis, unlike other photosynthetic bacteria such as purple and green sulfur bacteria. Fossilized filamentous algae from the Vindhya basin have been dated back to 1.6 to 1.7 billion years ago.[12]
Because of the wide range of types of algae, they have increasing different industrial and traditional applications in human society. Traditional seaweed farming practices have existed for thousands of years and have strong traditions in East Asia food cultures. More modern algaculture applications extend the food traditions for other applications include cattle feed, using algae for bioremediation or pollution control, transforming sunlight into algae fuels or other chemicals used in industrial processes, and in medical and scientific applications. A 2020 review found that these applications of algae could play an important role in carbon sequestration in order to mitigate climate change while providing valuable value-add products for global economies.[13]
Etymology and study
The singular alga is the Latin word for 'seaweed' and retains that meaning in English.[14] The etymology is obscure. Although some speculate that it is related to Latin algēre, 'be cold',[15] no reason is known to associate seaweed with temperature. A more likely source is alliga, 'binding, entwining'.[16]
The Ancient Greek word for 'seaweed' was φῦκος (phŷkos), which could mean either the seaweed (probably red algae) or a red dye derived from it. The Latinization, fūcus, meant primarily the cosmetic rouge. The etymology is uncertain, but a strong candidate has long been some word related to the Biblical פוך (pūk), 'paint' (if not that word itself), a cosmetic eye-shadow used by the ancient Egyptians and other inhabitants of the eastern Mediterranean. It could be any color: black, red, green, or blue.[17]
Accordingly, the modern study of marine and freshwater algae is called either phycology or algology, depending on whether the Greek or Latin root is used. The name fucus appears in a number of taxa.
Classifications
The committee on the International Code of Botanical Nomenclature has recommended certain suffixes for use in the classification of algae. These are -phyta for division, -phyceae for class, -phycideae for subclass, -ales for order, -inales for suborder, -aceae for family, -oidease for subfamily, a Greek-based name for genus, and a Latin-based name for species.
Algal characteristics basic to primary classification
The primary classification of algae is based on certain morphological features. The chief among these are (a) pigment constitution of the cell, (b) chemical nature of stored food materials, (c) kind, number, point of insertion and relative length of the flagella on the motile cell, (d) chemical composition of cell wall and (e) presence or absence of a definitely organized nucleus in the cell or any other significant details of cell structure.
History of classification of algae
Although Carolus Linnaeus (1754) included algae along with lichens in his 25th class Cryptogamia, he did not elaborate further on the classification of algae.
Jean Pierre Étienne Vaucher (1803) was perhaps the first to propose a system of classification of algae, and he recognized three groups, Conferves, Ulves, and Tremelles. While Johann Heinrich Friedrich Link (1820) classified algae on the basis of the colour of the pigment and structure, William Henry Harvey (1836) proposed a system of classification on the basis of the habitat and the pigment. J. G. Agardh (1849–1898) divided algae into six orders: Diatomaceae, Nostochineae, Confervoideae, Ulvaceae, Floriadeae and Fucoideae. Around 1880, algae along with fungi were grouped under Thallophyta, a division created by Eichler (1836). Encouraged by this, Adolf Engler and Karl A. E. Prantl (1912) proposed a revised scheme of classification of algae and included fungi in algae as they were of opinion that fungi have been derived from algae. The scheme proposed by Engler and Prantl is summarised as follows:[18]
- Schizophyta
- Phytosarcodina
- Flagellata
- Dinoflagellata
- Bacillariophyta
- Conjugatae
- Chlorophyceae
- Charophyta
- Phaeophyceae
- Rhodophyceae
- Eumycetes (Fungi)
The algae contain chloroplasts that are similar in structure to cyanobacteria. Chloroplasts contain circular DNA like that in cyanobacteria and are interpreted as representing reduced endosymbiotic cyanobacteria. However, the exact origin of the chloroplasts is different among separate lineages of algae, reflecting their acquisition during different endosymbiotic events. The table below describes the composition of the three major groups of algae. Their lineage relationships are shown in the figure in the upper right. Many of these groups contain some members that are no longer photosynthetic. Some retain plastids, but not chloroplasts, while others have lost plastids entirely.
Phylogeny based on plastid[19] not nucleocytoplasmic genealogy:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Supergroup affiliation | Members | Endosymbiont | Summary |
|---|---|---|---|
| Primoplantae/ Archaeplastida |
|
Cyanobacteria | These algae have "primary" chloroplasts, i.e. the chloroplasts are surrounded by two membranes and probably developed through a single endosymbiotic event. The chloroplasts of red algae have chlorophylls a and c (often), and phycobilins, while those of green algae have chloroplasts with chlorophyll a and b without phycobilins. Land plants are pigmented similarly to green algae and probably developed from them, thus the Chlorophyta is a sister taxon to the plants; sometimes the Chlorophyta, the Charophyta, and land plants are grouped together as the Viridiplantae. |
| Excavata and Rhizaria |
|
Green algae |
These groups have green chloroplasts containing chlorophylls a and b.[20] Their chloroplasts are surrounded by four and three membranes, respectively, and were probably retained from ingested green algae. Chlorarachniophytes, which belong to the phylum Cercozoa, contain a small nucleomorph, which is a relict of the algae's nucleus. Euglenids, which belong to the phylum Euglenozoa, live primarily in fresh water and have chloroplasts with only three membranes. The endosymbiotic green algae may have been acquired through myzocytosis rather than phagocytosis.[21] |
| Halvaria and Hacrobia |
|
Red algae |
These groups have chloroplasts containing chlorophylls a and c, and phycobilins. The shape can vary; they may be of discoid, plate-like, reticulate, cup-shaped, spiral, or ribbon shaped. They have one or more pyrenoids to preserve protein and starch. The latter chlorophyll type is not known from any prokaryotes or primary chloroplasts, but genetic similarities with red algae suggest a relationship there.[22] In the first three of these groups (Chromista), the chloroplast has four membranes, retaining a nucleomorph in cryptomonads, and they likely share a common pigmented ancestor, although other evidence casts doubt on whether the heterokonts, Haptophyta, and cryptomonads are in fact more closely related to each other than to other groups.[23][24] The typical dinoflagellate chloroplast has three membranes, but considerable diversity exists in chloroplasts within the group, and a number of endosymbiotic events apparently occurred.[6] The Apicomplexa, a group of closely related parasites, also have plastids called apicoplasts, which are not photosynthetic, but appear to have a common origin with dinoflagellate chloroplasts.[6] |
.png.webp)
Linnaeus, in Species Plantarum (1753),[25] the starting point for modern botanical nomenclature, recognized 14 genera of algae, of which only four are currently considered among algae.[26] In Systema Naturae, Linnaeus described the genera Volvox and Corallina, and a species of Acetabularia (as Madrepora), among the animals.
In 1768, Samuel Gottlieb Gmelin (1744–1774) published the Historia Fucorum, the first work dedicated to marine algae and the first book on marine biology to use the then new binomial nomenclature of Linnaeus. It included elaborate illustrations of seaweed and marine algae on folded leaves.[27][28]
W. H. Harvey (1811–1866) and Lamouroux (1813)[29] were the first to divide macroscopic algae into four divisions based on their pigmentation. This is the first use of a biochemical criterion in plant systematics. Harvey's four divisions are: red algae (Rhodospermae), brown algae (Melanospermae), green algae (Chlorospermae), and Diatomaceae.[30][31]
At this time, microscopic algae were discovered and reported by a different group of workers (e.g., O. F. Müller and Ehrenberg) studying the Infusoria (microscopic organisms). Unlike macroalgae, which were clearly viewed as plants, microalgae were frequently considered animals because they are often motile.[29] Even the nonmotile (coccoid) microalgae were sometimes merely seen as stages of the lifecycle of plants, macroalgae, or animals.[32][33]
Although used as a taxonomic category in some pre-Darwinian classifications, e.g., Linnaeus (1753), de Jussieu (1789), Horaninow (1843), Agassiz (1859), Wilson & Cassin (1864), in further classifications, the "algae" are seen as an artificial, polyphyletic group.
Throughout the 20th century, most classifications treated the following groups as divisions or classes of algae: cyanophytes, rhodophytes, chrysophytes, xanthophytes, bacillariophytes, phaeophytes, pyrrhophytes (cryptophytes and dinophytes), euglenophytes, and chlorophytes. Later, many new groups were discovered (e.g., Bolidophyceae), and others were splintered from older groups: charophytes and glaucophytes (from chlorophytes), many heterokontophytes (e.g., synurophytes from chrysophytes, or eustigmatophytes from xanthophytes), haptophytes (from chrysophytes), and chlorarachniophytes (from xanthophytes).
With the abandonment of plant-animal dichotomous classification, most groups of algae (sometimes all) were included in Protista, later also abandoned in favour of Eukaryota. However, as a legacy of the older plant life scheme, some groups that were also treated as protozoans in the past still have duplicated classifications (see ambiregnal protists).
Some parasitic algae (e.g., the green algae Prototheca and Helicosporidium, parasites of metazoans, or Cephaleuros, parasites of plants) were originally classified as fungi, sporozoans, or protistans of incertae sedis,[34] while others (e.g., the green algae Phyllosiphon and Rhodochytrium, parasites of plants, or the red algae Pterocladiophila and Gelidiocolax mammillatus, parasites of other red algae, or the dinoflagellates Oodinium, parasites of fish) had their relationship with algae conjectured early. In other cases, some groups were originally characterized as parasitic algae (e.g., Chlorochytrium), but later were seen as endophytic algae.[35] Some filamentous bacteria (e.g., Beggiatoa) were originally seen as algae. Furthermore, groups like the apicomplexans are also parasites derived from ancestors that possessed plastids, but are not included in any group traditionally seen as algae.
Relationship to land plants
The first land plants probably evolved from shallow freshwater charophyte algae much like Chara almost 500 million years ago. These probably had an isomorphic alternation of generations and were probably filamentous. Fossils of isolated land plant spores suggest land plants may have been around as long as 475 million years ago.[36][37]
Morphology

A range of algal morphologies is exhibited, and convergence of features in unrelated groups is common. The only groups to exhibit three-dimensional multicellular thalli are the reds and browns, and some chlorophytes.[38] Apical growth is constrained to subsets of these groups: the florideophyte reds, various browns, and the charophytes.[38] The form of charophytes is quite different from those of reds and browns, because they have distinct nodes, separated by internode 'stems'; whorls of branches reminiscent of the horsetails occur at the nodes.[38] Conceptacles are another polyphyletic trait; they appear in the coralline algae and the Hildenbrandiales, as well as the browns.[38]
Most of the simpler algae are unicellular flagellates or amoeboids, but colonial and nonmotile forms have developed independently among several of the groups. Some of the more common organizational levels, more than one of which may occur in the lifecycle of a species, are
- Colonial: small, regular groups of motile cells
- Capsoid: individual non-motile cells embedded in mucilage
- Coccoid: individual non-motile cells with cell walls
- Palmelloid: nonmotile cells embedded in mucilage
- Filamentous: a string of connected nonmotile cells, sometimes branching
- Parenchymatous: cells forming a thallus with partial differentiation of tissues
In three lines, even higher levels of organization have been reached, with full tissue differentiation. These are the brown algae,[39]—some of which may reach 50 m in length (kelps)[40]—the red algae,[41] and the green algae.[42] The most complex forms are found among the charophyte algae (see Charales and Charophyta), in a lineage that eventually led to the higher land plants. The innovation that defines these nonalgal plants is the presence of female reproductive organs with protective cell layers that protect the zygote and developing embryo. Hence, the land plants are referred to as the Embryophytes.
Turfs
The term algal turf is commonly used but poorly defined. Algal turfs are thick, carpet-like beds of seaweed that retain sediment and compete with foundation species like corals and kelps, and they are usually less than 15 cm tall. Such a turf may consist of one or more species, and will generally cover an area in the order of a square metre or more. Some common characteristics are listed:[43]
- Algae that form aggregations that have been described as turfs include diatoms, cyanobacteria, chlorophytes, phaeophytes and rhodophytes. Turfs are often composed of numerous species at a wide range of spatial scales, but monospecific turfs are frequently reported.[43]
- Turfs can be morphologically highly variable over geographic scales and even within species on local scales and can be difficult to identify in terms of the constituent species.[43]
- Turfs have been defined as short algae, but this has been used to describe height ranges from less than 0.5 cm to more than 10 cm. In some regions, the descriptions approached heights which might be described as canopies (20 to 30 cm).[43]
Physiology
Many algae, particularly members of the Characeae species,[44] have served as model experimental organisms to understand the mechanisms of the water permeability of membranes, osmoregulation, turgor regulation, salt tolerance, cytoplasmic streaming, and the generation of action potentials.
Phytohormones are found not only in higher plants, but in algae, too.[45]
Symbiotic algae
Some species of algae form symbiotic relationships with other organisms. In these symbioses, the algae supply photosynthates (organic substances) to the host organism providing protection to the algal cells. The host organism derives some or all of its energy requirements from the algae. Examples are:
Lichens
.jpg.webp)
Lichens are defined by the International Association for Lichenology to be "an association of a fungus and a photosynthetic symbiont resulting in a stable vegetative body having a specific structure".[46] The fungi, or mycobionts, are mainly from the Ascomycota with a few from the Basidiomycota. In nature, they do not occur separate from lichens. It is unknown when they began to associate.[47] One mycobiont associates with the same phycobiont species, rarely two, from the green algae, except that alternatively, the mycobiont may associate with a species of cyanobacteria (hence "photobiont" is the more accurate term). A photobiont may be associated with many different mycobionts or may live independently; accordingly, lichens are named and classified as fungal species.[48] The association is termed a morphogenesis because the lichen has a form and capabilities not possessed by the symbiont species alone (they can be experimentally isolated). The photobiont possibly triggers otherwise latent genes in the mycobiont.[49]
Trentepohlia is an example of a common green alga genus worldwide that can grow on its own or be lichenised. Lichen thus share some of the habitat and often similar appearance with specialized species of algae (aerophytes) growing on exposed surfaces such as tree trunks and rocks and sometimes discoloring them.
Coral reefs

Coral reefs are accumulated from the calcareous exoskeletons of marine invertebrates of the order Scleractinia (stony corals). These animals metabolize sugar and oxygen to obtain energy for their cell-building processes, including secretion of the exoskeleton, with water and carbon dioxide as byproducts. Dinoflagellates (algal protists) are often endosymbionts in the cells of the coral-forming marine invertebrates, where they accelerate host-cell metabolism by generating sugar and oxygen immediately available through photosynthesis using incident light and the carbon dioxide produced by the host. Reef-building stony corals (hermatypic corals) require endosymbiotic algae from the genus Symbiodinium to be in a healthy condition.[50] The loss of Symbiodinium from the host is known as coral bleaching, a condition which leads to the deterioration of a reef.
Sea sponges
Endosymbiontic green algae live close to the surface of some sponges, for example, breadcrumb sponges (Halichondria panicea). The alga is thus protected from predators; the sponge is provided with oxygen and sugars which can account for 50 to 80% of sponge growth in some species.[51]
Lifecycle
Rhodophyta, Chlorophyta, and Heterokontophyta, the three main algal divisions, have lifecycles which show considerable variation and complexity. In general, an asexual phase exists where the seaweed's cells are diploid, a sexual phase where the cells are haploid, followed by fusion of the male and female gametes. Asexual reproduction permits efficient population increases, but less variation is possible. Commonly, in sexual reproduction of unicellular and colonial algae, two specialized, sexually compatible, haploid gametes make physical contact and fuse to form a zygote. To ensure a successful mating, the development and release of gametes is highly synchronized and regulated; pheromones may play a key role in these processes.[52] Sexual reproduction allows for more variation and provides the benefit of efficient recombinational repair of DNA damages during meiosis, a key stage of the sexual cycle.[53] However, sexual reproduction is more costly than asexual reproduction.[54] Meiosis has been shown to occur in many different species of algae.[55]
Numbers

The Algal Collection of the US National Herbarium (located in the National Museum of Natural History) consists of approximately 320,500 dried specimens, which, although not exhaustive (no exhaustive collection exists), gives an idea of the order of magnitude of the number of algal species (that number remains unknown).[56] Estimates vary widely. For example, according to one standard textbook,[57] in the British Isles the UK Biodiversity Steering Group Report estimated there to be 20,000 algal species in the UK. Another checklist reports only about 5,000 species. Regarding the difference of about 15,000 species, the text concludes: "It will require many detailed field surveys before it is possible to provide a reliable estimate of the total number of species ..."
Regional and group estimates have been made, as well:
- 5,000–5,500 species of red algae worldwide
- "some 1,300 in Australian Seas"[58]
- 400 seaweed species for the western coastline of South Africa,[59] and 212 species from the coast of KwaZulu-Natal.[60] Some of these are duplicates, as the range extends across both coasts, and the total recorded is probably about 500 species. Most of these are listed in List of seaweeds of South Africa. These exclude phytoplankton and crustose corallines.
- 669 marine species from California (US)[61]
- 642 in the check-list of Britain and Ireland[62]
and so on, but lacking any scientific basis or reliable sources, these numbers have no more credibility than the British ones mentioned above. Most estimates also omit microscopic algae, such as phytoplankton.
The most recent estimate suggests 72,500 algal species worldwide.[63]
Distribution
The distribution of algal species has been fairly well studied since the founding of phytogeography in the mid-19th century.[64] Algae spread mainly by the dispersal of spores analogously to the dispersal of Plantae by seeds and spores. This dispersal can be accomplished by air, water, or other organisms. Due to this, spores can be found in a variety of environments: fresh and marine waters, air, soil, and in or on other organisms.[64] Whether a spore is to grow into an organism depends on the combination of the species and the environmental conditions where the spore lands.
The spores of freshwater algae are dispersed mainly by running water and wind, as well as by living carriers.[64] However, not all bodies of water can carry all species of algae, as the chemical composition of certain water bodies limits the algae that can survive within them.[64] Marine spores are often spread by ocean currents. Ocean water presents many vastly different habitats based on temperature and nutrient availability, resulting in phytogeographic zones, regions, and provinces.[65]
To some degree, the distribution of algae is subject to floristic discontinuities caused by geographical features, such as Antarctica, long distances of ocean or general land masses. It is, therefore, possible to identify species occurring by locality, such as "Pacific algae" or "North Sea algae". When they occur out of their localities, hypothesizing a transport mechanism is usually possible, such as the hulls of ships. For example, Ulva reticulata and U. fasciata travelled from the mainland to Hawaii in this manner.
Mapping is possible for select species only: "there are many valid examples of confined distribution patterns."[66] For example, Clathromorphum is an arctic genus and is not mapped far south of there.[67] However, scientists regard the overall data as insufficient due to the "difficulties of undertaking such studies."[68]
Ecology

Algae are prominent in bodies of water, common in terrestrial environments, and are found in unusual environments, such as on snow and ice. Seaweeds grow mostly in shallow marine waters, under 100 m (330 ft) deep; however, some such as Navicula pennata have been recorded to a depth of 360 m (1,180 ft).[69] A type of algae, Ancylonema nordenskioeldii, was found in Greenland in areas known as the 'Dark Zone', which caused an increase in the rate of melting ice sheet.[70] Same algae was found in the Italian Alps, after pink ice appeared on parts of the Presena glacier.[71]
The various sorts of algae play significant roles in aquatic ecology. Microscopic forms that live suspended in the water column (phytoplankton) provide the food base for most marine food chains. In very high densities (algal blooms), these algae may discolor the water and outcompete, poison, or asphyxiate other life forms.
Algae can be used as indicator organisms to monitor pollution in various aquatic systems.[72] In many cases, algal metabolism is sensitive to various pollutants. Due to this, the species composition of algal populations may shift in the presence of chemical pollutants.[72] To detect these changes, algae can be sampled from the environment and maintained in laboratories with relative ease.[72]
On the basis of their habitat, algae can be categorized as: aquatic (planktonic, benthic, marine, freshwater, lentic, lotic),[73] terrestrial, aerial (subaerial),[74] lithophytic, halophytic (or euryhaline), psammon, thermophilic, cryophilic, epibiont (epiphytic, epizoic), endosymbiont (endophytic, endozoic), parasitic, calcifilic or lichenic (phycobiont).[75]
Cultural associations
In classical Chinese, the word 藻 is used both for "algae" and (in the modest tradition of the imperial scholars) for "literary talent". The third island in Kunming Lake beside the Summer Palace in Beijing is known as the Zaojian Tang Dao, which thus simultaneously means "Island of the Algae-Viewing Hall" and "Island of the Hall for Reflecting on Literary Talent".
Cultivation
Algaculture is a form of aquaculture involving the farming of species of algae.
The majority of algae that are intentionally cultivated fall into the category of microalgae (also referred to as phytoplankton, microphytes, or planktonic algae). Macroalgae, commonly known as seaweed, also have many commercial and industrial uses, but due to their size and the specific requirements of the environment in which they need to grow, they do not lend themselves as readily to cultivation (this may change, however, with the advent of newer seaweed cultivators, which are basically algae scrubbers using upflowing air bubbles in small containers).
Commercial and industrial algae cultivation has numerous uses, including production of food ingredients such as omega-3 fatty acids or natural food colorants and dyes, food, fertilizer, bioplastics, chemical feedstock (raw material), pharmaceuticals, and algal fuel, and can also be used as a means of pollution control.
Global production of farmed aquatic plants, overwhelmingly dominated by seaweeds, grew in output volume from 13.5 million tonnes in 1995 to just over 30 million tonnes in 2016.[76]Seaweed farming
.jpg.webp)

Seaweed farming or kelp farming is the practice of cultivating and harvesting seaweed. In its simplest form, it consists of the management of naturally found batches. In its most advanced form, it consists of fully controlling the life cycle of the algae.
The top seven most cultivated seaweed taxa are Eucheuma spp., Kappaphycus alvarezii, Gracilaria spp., Saccharina japonica, Undaria pinnatifida, Pyropia spp., and Sargassum fusiforme. Eucheuma and K. alvarezii are farmed for carrageenan (a gelling agent); Gracilaria is farmed for agar; while the rest are farmed for food. The largest seaweed-producing countries are China, Indonesia, and the Philippines. Other notable producers include South Korea, North Korea, Japan, Malaysia, and Zanzibar (Tanzania).[77] Seaweed farming has frequently been developed as an alternative to improve economic conditions and to reduce fishing pressure and overexploited fisheries.[78]
Global production of farmed aquatic plants, overwhelmingly dominated by seaweeds, grew in output volume from 13.5×106 t (13,300,000 long tons; 14,900,000 short tons) in 1995 to just over 30×106 t (30,000,000 long tons; 33,000,000 short tons) in 2016.[79] As of 2014, seaweed was 27% of all marine aquaculture.[80] Seaweed farming is a carbon negative crop, with a high potential for climate change mitigation .[80] The IPCC Special Report on the Ocean and Cryosphere in a Changing Climate recommends "further research attention" as a mitigation tactic.[81]Bioreactors
Uses

Agar
Agar, a gelatinous substance derived from red algae, has a number of commercial uses.[82] It is a good medium on which to grow bacteria and fungi, as most microorganisms cannot digest agar.
Alginates
Alginic acid, or alginate, is extracted from brown algae. Its uses range from gelling agents in food, to medical dressings. Alginic acid also has been used in the field of biotechnology as a biocompatible medium for cell encapsulation and cell immobilization. Molecular cuisine is also a user of the substance for its gelling properties, by which it becomes a delivery vehicle for flavours.
Between 100,000 and 170,000 wet tons of Macrocystis are harvested annually in New Mexico for alginate extraction and abalone feed.[83][84]
Energy source
To be competitive and independent from fluctuating support from (local) policy on the long run, biofuels should equal or beat the cost level of fossil fuels. Here, algae-based fuels hold great promise,[85][86] directly related to the potential to produce more biomass per unit area in a year than any other form of biomass. The break-even point for algae-based biofuels is estimated to occur by 2025.[87]
Fertilizer

For centuries, seaweed has been used as a fertilizer; George Owen of Henllys writing in the 16th century referring to drift weed in South Wales:[88]
This kind of ore they often gather and lay on great heapes, where it heteth and rotteth, and will have a strong and loathsome smell; when being so rotten they cast on the land, as they do their muck, and thereof springeth good corn, especially barley ... After spring-tydes or great rigs of the sea, they fetch it in sacks on horse backes, and carie the same three, four, or five miles, and cast it on the lande, which doth very much better the ground for corn and grass.
Today, algae are used by humans in many ways; for example, as fertilizers, soil conditioners, and livestock feed.[89] Aquatic and microscopic species are cultured in clear tanks or ponds and are either harvested or used to treat effluents pumped through the ponds. Algaculture on a large scale is an important type of aquaculture in some places. Maerl is commonly used as a soil conditioner.
Nutrition

Naturally growing seaweeds are an important source of food, especially in Asia, leading some to label them as superfoods.[90] They provide many vitamins including: A, B1, B2, B6, niacin, and C, and are rich in iodine, potassium, iron, magnesium, and calcium.[91] In addition, commercially cultivated microalgae, including both algae and cyanobacteria, are marketed as nutritional supplements, such as spirulina,[92] Chlorella and the vitamin-C supplement from Dunaliella, high in beta-carotene.
Algae are national foods of many nations: China consumes more than 70 species, including fat choy, a cyanobacterium considered a vegetable; Japan, over 20 species such as nori and aonori;[93] Ireland, dulse; Chile, cochayuyo.[94] Laver is used to make laver bread in Wales, where it is known as bara lawr; in Korea, gim. It is also used along the west coast of North America from California to British Columbia, in Hawaii and by the Māori of New Zealand. Sea lettuce and badderlocks are salad ingredients in Scotland, Ireland, Greenland, and Iceland. Algae is being considered a potential solution for world hunger problem.[95][96][97]
Two popular forms of algae are used in cuisine:
- Chlorella: This form of alga is found in freshwater and contains photosynthetic pigments in its chloroplast. It is high in iron, zinc, magnesium, vitamin B2 and Omega-3 Fatty acids.
Furthermore, it contains all nine of the essential amino acids the body does not produce on its own[98]
- Spirulina: Known otherwise as a cyanobacterium (a prokaryote, incorrectly referred to as a "blue-green alga"), contains 10% more protein than Chlorella as well as more thiamine and copper.[99]
The oils from some algae have high levels of unsaturated fatty acids. For example, Parietochloris incisa is very high in arachidonic acid, where it reaches up to 47% of the triglyceride pool.[100] Some varieties of algae favored by vegetarianism and veganism contain the long-chain, essential omega-3 fatty acids, docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA). Fish oil contains the omega-3 fatty acids, but the original source is algae (microalgae in particular), which are eaten by marine life such as copepods and are passed up the food chain.[101] Algae have emerged in recent years as a popular source of omega-3 fatty acids for vegetarians who cannot get long-chain EPA and DHA from other vegetarian sources such as flaxseed oil, which only contains the short-chain alpha-linolenic acid (ALA).
Pollution control
- Sewage can be treated with algae,[102] reducing the use of large amounts of toxic chemicals that would otherwise be needed.
- Algae can be used to capture fertilizers in runoff from farms. When subsequently harvested, the enriched algae can be used as fertilizer.
- Aquaria and ponds can be filtered using algae, which absorb nutrients from the water in a device called an algae scrubber, also known as an algae turf scrubber.[103][104][105][106]
Agricultural Research Service scientists found that 60–90% of nitrogen runoff and 70–100% of phosphorus runoff can be captured from manure effluents using a horizontal algae scrubber, also called an algal turf scrubber (ATS). Scientists developed the ATS, which consists of shallow, 100-foot raceways of nylon netting where algae colonies can form, and studied its efficacy for three years. They found that algae can readily be used to reduce the nutrient runoff from agricultural fields and increase the quality of water flowing into rivers, streams, and oceans. Researchers collected and dried the nutrient-rich algae from the ATS and studied its potential as an organic fertilizer. They found that cucumber and corn seedlings grew just as well using ATS organic fertilizer as they did with commercial fertilizers.[107] Algae scrubbers, using bubbling upflow or vertical waterfall versions, are now also being used to filter aquaria and ponds.
Polymers
Various polymers can be created from algae, which can be especially useful in the creation of bioplastics. These include hybrid plastics, cellulose-based plastics, poly-lactic acid, and bio-polyethylene.[108] Several companies have begun to produce algae polymers commercially, including for use in flip-flops[109] and in surf boards.[110]
Bioremediation
The alga Stichococcus bacillaris has been seen to colonize silicone resins used at archaeological sites; biodegrading the synthetic substance.[111]
Pigments
The natural pigments (carotenoids and chlorophylls) produced by algae can be used as alternatives to chemical dyes and coloring agents.[112] The presence of some individual algal pigments, together with specific pigment concentration ratios, are taxon-specific: analysis of their concentrations with various analytical methods, particularly high-performance liquid chromatography, can therefore offer deep insight into the taxonomic composition and relative abundance of natural algae populations in sea water samples.[113][114]
Stabilizing substances
Carrageenan, from the red alga Chondrus crispus, is used as a stabilizer in milk products.
Additional images
 Algae bladder
Algae bladder
See also
- AlgaeBase
- AlgaePARC
- Eutrophication
- Iron fertilization
- Marimo algae
- Microbiofuels
- Microphyte
- Photobioreactor
- Phycotechnology
- Plant
- Toxoid – anatoxin
References
- Butterfield, N. J. (2000). "Bangiomorpha pubescens n. gen., n. sp.: Implications for the evolution of sex, multicellularity, and the Mesoproterozoic/Neoproterozoic radiation of eukaryotes". Paleobiology. 26 (3): 386–404. doi:10.1666/0094-8373(2000)026<0386:BPNGNS>2.0.CO;2. ISSN 0094-8373. S2CID 36648568. Archived from the original on 7 March 2007.
- T.M. Gibson (2018). "Precise age of Bangiomorpha pubescens dates the origin of eukaryotic photosynthesis". Geology. 46 (2): 135–138. Bibcode:2018Geo....46..135G. doi:10.1130/G39829.1.
- Lee, R. E. (2008). Phycology. Cambridge University Press. ISBN 9780521367448.
- Nabors, Murray W. (2004). Introduction to Botany. San Francisco: Pearson Education, Inc. ISBN 978-0-8053-4416-5.
- Allaby, M., ed. (1992). "Algae". The Concise Dictionary of Botany. Oxford University Press.
- Keeling, Patrick J. (2004). "Diversity and evolutionary history of plastids and their hosts". American Journal of Botany. 91 (10): 1481–1493. doi:10.3732/ajb.91.10.1481. PMID 21652304.
- Palmer, J. D.; Soltis, D. E.; Chase, M. W. (2004). "The plant tree of life: an overview and some points of view". American Journal of Botany. 91 (10): 1437–1445. doi:10.3732/ajb.91.10.1437. PMID 21652302.
- Smithsonian National Museum of Natural History; Department of Botany. "Algae Research". Archived from the original on 2 July 2010. Retrieved 25 August 2010.
- Pringsheim, E. G. 1963. Farblose Algen. Ein beitrag zur Evolutionsforschung. Gustav Fischer Verlag, Stuttgart. 471 pp., species:Algae#Pringsheim (1963).
- Tartar, A.; Boucias, D. G.; Becnel, J. J.; Adams, B. J. (2003). "Comparison of plastid 16S rRNA (rrn 16) genes from Helicosporidium spp.: Evidence supporting the reclassification of Helicosporidia as green algae (Chlorophyta)". International Journal of Systematic and Evolutionary Microbiology. 53 (Pt 6): 1719–1723. doi:10.1099/ijs.0.02559-0. PMID 14657099.
- Figueroa‐Martinez, F.; Nedelcu, A. M.; Smith, D. R.; Reyes‐Prieto, A. (2015). "When the lights go out: the evolutionary fate of free‐living colorless green algae". New Phytologist. 206 (3): 972–982. doi:10.1111/nph.13279. PMC 5024002. PMID 26042246.
- Bengtson, S.; Belivanova, V.; Rasmussen, B.; Whitehouse, M. (2009). "The controversial 'Cambrian' fossils of the Vindhyan are real but more than a billion years older". Proceedings of the National Academy of Sciences of the United States of America. 106 (19): 7729–7734. Bibcode:2009PNAS..106.7729B. doi:10.1073/pnas.0812460106. PMC 2683128. PMID 19416859.
- Paul, Vishal; Chandra Shekharaiah, P. S.; Kushwaha, Shivbachan; Sapre, Ajit; Dasgupta, Santanu; Sanyal, Debanjan (2020). Deb, Dipankar; Dixit, Ambesh; Chandra, Laltu (eds.). "Role of Algae in CO2 Sequestration Addressing Climate Change: A Review". Renewable Energy and Climate Change. Smart Innovation, Systems and Technologies. Singapore: Springer. 161: 257–265. doi:10.1007/978-981-32-9578-0_23. ISBN 978-981-329-578-0. S2CID 202902934.
- "alga, algae". Webster's Third New International Dictionary of the English Language Unabridged with Seven Language Dictionary. Vol. 1. Encyclopædia Britannica, Inc. 1986.
- Partridge, Eric (1983). "algae". Origins. ISBN 9780517414255.
- Lewis, Charlton T.; Short, Charles (1879). "Alga". A Latin Dictionary. Oxford: Clarendon Press. Retrieved 31 December 2017.
- Cheyne, Thomas Kelly; Black, John Sutherland (1902). Encyclopædia biblica: A critical dictionary of the literary, political and religious history, the archæology, geography, and natural history of the Bible. Macmillan Company. p. 3525.
- B. R. Vashishta Revised by A. K. Sinha and V. P. Singh (1960). Botany for degree students algae. New Delhi: S. Chand and Company.
- Bhattacharya, D.; Medlin, L. (1998). "Algal Phylogeny and the Origin of Land Plants" (PDF). Plant Physiology. 116 (1): 9–15. doi:10.1104/pp.116.1.9. PMC 1539170. Archived (PDF) from the original on 7 February 2009.
- Losos, Jonathan B.; Mason, Kenneth A.; Singer, Susan R. (2007). Biology (8 ed.). McGraw-Hill. ISBN 978-0-07-304110-0.
- Archibald, J. M.; Keeling, P. J. (November 2002). "Recycled plastids: A 'green movement' in eukaryotic evolution". Trends in Genetics. 18 (11): 577–584. doi:10.1016/S0168-9525(02)02777-4. PMID 12414188.
- Janson, Sven; Graneli, Edna (September 2003). "Genetic analysis of the psbA gene from single cells indicates a cryptomonad origin of the plastid in Dinophysis (Dinophyceae)". Phycologia. 42 (5): 473–477. doi:10.2216/i0031-8884-42-5-473.1. ISSN 0031-8884. S2CID 86730888.
- Wegener Parfrey, Laura; Barbero, Erika; Lasser, Elyse; Dunthorn, Micah; Bhattacharya, Debashish; Patterson, David J.; Katz, Laura A (December 2006). "Evaluating Support for the Current Classification of Eukaryotic Diversity". PLOS Genetics. 2 (12): e220. doi:10.1371/journal.pgen.0020220. PMC 1713255. PMID 17194223.
- Burki, F.; Shalchian-Tabrizi, K.; Minge, M.; Skjæveland, Å.; Nikolaev, S. I.; et al. (2007). Butler, Geraldine (ed.). "Phylogenomics Reshuffles the Eukaryotic Supergroups". PLOS ONE. 2 (8): e790. Bibcode:2007PLoSO...2..790B. doi:10.1371/journal.pone.0000790. PMC 1949142. PMID 17726520.
- Linnæus, Caroli (1753). Species Plantarum. Vol. 2. Impensis Laurentii Salvii. p. 1131.
- Sharma, O. P. (1 January 1986). Textbook of Algae. p. 22. ISBN 9780074519288.
- Gmelin, S. G. (1768). Historia Fucorum. St. Petersburg: Ex typographia Academiae scientiarum – via Google Books.
- Silva, P. C.; Basson, P. W.; Moe, R. L. (1996). Catalogue of the Benthic Marine Algae of the Indian Ocean. ISBN 9780520915817 – via Google Books.
- Medlin, Linda K.; Kooistra, Wiebe H. C. F.; Potter, Daniel; Saunders, Gary W.; Anderson, Robert A. (1997). "Phylogenetic relationships of the 'golden algae' (haptophytes, heterokont chromophytes) and their plastids" (PDF). Plant Systematics and Evolution: 188. Archived (PDF) from the original on 5 October 2013.
- Dixon, P. S. (1973). Biology of the Rhodophyta. Edinburgh: Oliver & Boyd. p. 232. ISBN 978-0-05-002485-0.
- Harvey, D. (1836). "Algae" (PDF). In Mackay, J. T. (ed.). Flora hibernica comprising the Flowering Plants Ferns Characeae Musci Hepaticae Lichenes and Algae of Ireland arranged according to the natural system with a synopsis of the genera according to the Linnaean system. pp. 157–254. Archived (PDF) from the original on 9 October 2022. Retrieved 31 December 2017..
- Braun, A. Algarum unicellularium genera nova et minus cognita, praemissis observationibus de algis unicellularibus in genere (New and less known genera of unicellular algae, preceded by observations respecting unicellular algae in general) Archived 20 April 2016 at the Wayback Machine. Lipsiae, Apud W. Engelmann, 1855. Translation at: Lankester, E. & Busk, G. (eds.). Quarterly Journal of Microscopical Science, 1857, vol. 5, (17), 13–16 Archived 4 March 2016 at the Wayback Machine; (18), 90–96 Archived 5 March 2016 at the Wayback Machine; (19), 143–149 Archived 4 March 2016 at the Wayback Machine.
- Siebold, C. Th. v. "Ueber einzellige Pflanzen und Thiere (On unicellular plants and animals) Archived 26 November 2014 at the Wayback Machine". In: Siebold, C. Th. v. & Kölliker, A. (1849). Zeitschrift für wissenschaftliche Zoologie, Bd. 1, p. 270. Translation at: Lankester, E. & Busk, G. (eds.). Quarterly Journal of Microscopical Science, 1853, vol. 1, (2), 111–121 Archived 4 March 2016 at the Wayback Machine; (3), 195–206 Archived 4 March 2016 at the Wayback Machine.
- Williams, B. A.; Keeling, P. J. (2003). "Cryptic organelles in parasitic protists and fungi". In Littlewood, D. T. J. (ed.). The Evolution of Parasitism. London: Elsevier Academic Press. p. 46. ISBN 978-0-12-031754-7.
- Round (1981). pp. 398–400, Round, F. E. (8 March 1984). The Ecology of Algae. ISBN 9780521269063. Retrieved 6 February 2015..
- Noble, Ivan (18 September 2003). "When plants conquered land". BBC. Archived from the original on 11 November 2006.
- Wellman, C. H.; Osterloff, P. L.; Mohiuddin, U. (2003). "Fragments of the earliest land plants". Nature. 425 (6955): 282–285. Bibcode:2003Natur.425..282W. doi:10.1038/nature01884. PMID 13679913. S2CID 4383813. Archived from the original on 30 August 2017.
- Xiao, S.; Knoll, A. H.; Yuan, X.; Pueschel, C. M. (2004). "Phosphatized multicellular algae in the Neoproterozoic Doushantuo Formation, China, and the early evolution of florideophyte red algae". American Journal of Botany. 91 (2): 214–227. doi:10.3732/ajb.91.2.214. PMID 21653378.
- Waggoner, Ben (1994–2008). "Introduction to the Phaeophyta: Kelps and brown "Algae"". University of California Museum of Palaeontology (UCMP). Archived from the original on 21 December 2008. Retrieved 19 December 2008.
- Thomas, D. N. (2002). Seaweeds. London: The Natural History Museum. ISBN 978-0-565-09175-0.
- Waggoner, Ben (1994–2008). "Introduction to the Rhodophyta, the red 'algae'". University of California Museum of Palaeontology (UCMP). Archived from the original on 18 December 2008. Retrieved 19 December 2008.
- "Introduction to the Green Algae". berkeley.edu. Archived from the original on 13 February 2007. Retrieved 15 February 2007.
- Connell, Sean; Foster, M.S.; Airoldi, Laura (9 January 2014). "What are algal turfs? Towards a better description of turfs". Marine Ecology Progress Series. 495: 299–307. Bibcode:2014MEPS..495..299C. doi:10.3354/meps10513.
- Tazawa, Masashi (2010). "Sixty Years Research with Characean Cells: Fascinating Material for Plant Cell Biology". Progress in Botany 72. Progress in Botany. Vol. 72. Springer. pp. 5–34. doi:10.1007/978-3-642-13145-5_1. ISBN 978-3-642-13145-5. Retrieved 7 October 2012.
- Tarakhovskaya, E. R.; Maslov, Yu. I.; Shishova, M. F. (April 2007). "Phytohormones in algae". Russian Journal of Plant Physiology. 54 (2): 163–170. doi:10.1134/s1021443707020021. S2CID 27373543.
- Brodo, Irwin M.; Sharnoff, Sylvia Duran; Sharnoff, Stephen; Laurie-Bourque, Susan (2001). Lichens of North America. New Haven: Yale University Press. p. 8. ISBN 978-0-300-08249-4.
- Pearson, Lorentz C. (1995). The Diversity and Evolution of Plants. CRC Press. p. 221. ISBN 978-0-8493-2483-3.
- Brodo et al. (2001), p. 6: "A species of lichen collected anywhere in its range has the same lichen-forming fungus and, generally, the same photobiont. (A particular photobiont, though, may associate with scores of different lichen fungi)."
- Brodo et al. (2001), p. 8.
- Taylor, Dennis L. (1983). "The coral-algal symbiosis". In Goff, Lynda J. (ed.). Algal Symbiosis: A Continuum of Interaction Strategies. CUP Archive. pp. 19–20. ISBN 978-0-521-25541-7.
- Knight, Susan (Fall 2001). "Are There Sponges in Your Lake?" (PDF). Lake Tides. Wisconsin Lakes Partnership. 26 (4): 4–5. Archived from the original (PDF) on 2 July 2007. Retrieved 4 August 2007 – via UWSP.edu.
- Frenkel, J.; Vyverman, W.; Pohnert, G. (2014). "Pheromone signaling during sexual reproduction in algae". Plant J. 79 (4): 632–644. doi:10.1111/tpj.12496. PMID 24597605.
- Bernstein H; Byerly HC; Hopf FA; Michod RE (1985). "Genetic damage, mutation, and the evolution of sex". Science. 229 (4719): 1277–81. Bibcode:1985Sci...229.1277B. doi:10.1126/science.3898363. PMID 3898363,
- Otto, S. P. (2009). "The evolutionary enigma of sex". Am. Nat. 174 (Suppl 1): S1–S14. doi:10.1086/599084. PMID 19441962. S2CID 9250680. Archived from the original on 9 April 2017.
- Heywood, P.; Magee, P. T. (1976). "Meiosis in protists: Some structural and physiological aspects of meiosis in algae, fungi, and protozoa". Bacteriol Rev. 40 (1): 190–240. doi:10.1128/MMBR.40.1.190-240.1976. PMC 413949. PMID 773364.
- "Algae Herbarium". National Museum of Natural History, Department of Botany. 2008. Archived from the original on 1 December 2008. Retrieved 19 December 2008.
- John (2002), p. 1.
- Huisman (2000), p. 25.
- Stegenga (1997).
- Clerck, Olivier (2005). Guide to the seaweeds of KwaZulu-Natal. ISBN 978-90-72619-64-8.
- Abbott and Hollenberg (1976), p. 2.
- Hardy and Guiry (2006).
- Guiry, Michael D. (2012). "How Many Species of Algae Are There?". Journal of Phycology. 48 (5): 1057–1063. doi:10.1111/j.1529-8817.2012.01222.x. PMID 27011267. S2CID 30911529.
- Round, F. E. (1981). "Chapter 8, Dispersal, continuity and phytogeography". The ecology of algae. pp. 357–361. ISBN 9780521269063 – via Google Books.
- Round (1981), p. 362.
- Round (1981), p. 357.
- Round (1981), p. 371.
- Round (1981), p. 366.
- Round (1981), p. 176.
- "Greenland Has a Mysterious 'Dark Zone' — And It's Getting Even Darker". Space.com. 10 April 2018.
- "Alpine glacier turning pink due to algae that accelerates climate change, scientists say". Sky News. 6 July 2020.
- Omar, Wan Maznah Wan (December 2010). "Perspectives on the Use of Algae as Biological Indicators for Monitoring and Protecting Aquatic Environments, with Special Reference to Malaysian Freshwater Ecosystems". Trop Life Sci Res. 21 (2): 51–67. PMC 3819078. PMID 24575199.
- Necchi Jr., O. (ed.) (2016). River Algae. Springer, Necchi, Orlando J. R. (2 June 2016). River Algae. ISBN 9783319319841..
- Johansen, J. R. (2012). "The Diatoms: Applications for the Environmental and Earth Sciences". In Smol, J. P.; Stoermer, E. F. (eds.). Diatoms of aerial habitats (2nd ed.). Cambridge University Press. pp. 465–472. ISBN 9781139492621 – via Google Books.
- Sharma, O. P. (1986). pp. 2–6, .
- In brief, The State of World Fisheries and Aquaculture, 2018 (PDF). FAO. 2018.
- Buschmann, Alejandro H.; Camus, Carolina; Infante, Javier; Neori, Amir; Israel, Álvaro; Hernández-González, María C.; Pereda, Sandra V.; Gomez-Pinchetti, Juan Luis; Golberg, Alexander; Tadmor-Shalev, Niva; Critchley, Alan T. (2 October 2017). "Seaweed production: overview of the global state of exploitation, farming and emerging research activity". European Journal of Phycology. 52 (4): 391–406. doi:10.1080/09670262.2017.1365175. ISSN 0967-0262. S2CID 53640917.
- Ask, E.I (1990). Cottonii and Spinosum Cultivation Handbook. Philippines: FMC BioPolymer Corporation. p. 52.
- In brief, The State of World Fisheries and Aquaculture, 2018 (PDF). Food and Agriculture Organization. 2018.
- Duarte, Carlos M.; Wu, Jiaping; Xiao, Xi; Bruhn, Annette; Krause-Jensen, Dorte (2017). "Can Seaweed Farming Play a Role in Climate Change Mitigation and Adaptation?". Frontiers in Marine Science. 4. doi:10.3389/fmars.2017.00100. ISSN 2296-7745.
- Bindoff, N. L.; Cheung, W. W. L.; Kairo, J. G.; Arístegui, J.; et al. (2019). "Chapter 5: Changing Ocean, Marine Ecosystems, and Dependent Communities" (PDF). IPCC Special Report on the Ocean and Cryosphere in a Changing Climate. pp. 447–587.
- Lewis, J. G.; Stanley, N. F.; Guist, G. G. (1988). "9. Commercial production of algal hydrocolloides". In Lembi, C. A.; Waaland, J. R. (eds.). Algae and Human Affairs. Cambridge University Press. ISBN 978-0-521-32115-0.
- "Macrocystis C. Agardh 1820: 46". AlgaeBase. Archived from the original on 4 January 2009. Retrieved 28 December 2008.
- "Secondary Products of Brown Algae". Algae Research. Smithsonian National Museum of Natural History. Archived from the original on 13 April 2009. Retrieved 29 December 2008.
- Chisti, Y. (May–June 2007). "Biodiesel from microalgae". Biotechnology Advances. 25 (3): 294–306. doi:10.1016/j.biotechadv.2007.02.001. PMID 17350212.
- Yang, Z. K.; Niu, Y. F.; Ma, Y. H.; Xue, J.; Zhang, M. H.; Yang, W. D.; Liu, J. S.; Lu, S. H.; Guan, Y.; Li, H. Y. (4 May 2013). "Molecular and cellular mechanisms of neutral lipid accumulation in diatom following nitrogen deprivation". Biotechnology for Biofuels. 6 (1): 67. doi:10.1186/1754-6834-6-67. PMC 3662598. PMID 23642220.
- Wijffels, René H.; Barbosa, Maria J. (2010). "An Outlook on Microalgal Biofuels". Science. 329 (5993): 796–799. Bibcode:2010Sci...329..796W. doi:10.1126/science.1189003. PMID 20705853. S2CID 206526311.
- Read, Clare Sewell (1849). "On the Farming of South Wales: Prize Report". Journal of the Royal Agricultural Society of England. 10: 142–143.
- McHugh, Dennis J. (2003). "9, Other Uses of Seaweeds". A Guide to the Seaweed Industry: FAO Fisheries Technical Paper 441. Rome: Fisheries and Aquaculture Department, Food and Agriculture Organization (FAO) of the United Nations. ISBN 978-92-5-104958-7. Archived from the original on 28 December 2008.
- Jung, Frederich; Kruger-Genge, Anne; Kupper, J.-H.; Waldeck, P (April 2019). "Spirulina platensis, a super food?". ResearchGate. 5: 43. Retrieved 21 December 2020.
- Simoons, Frederick J. (1991). "6, Seaweeds and Other Algae". Food in China: A Cultural and Historical Inquiry. CRC Press. pp. 179–190. ISBN 978-0-936923-29-1.
- Morton, Steve L. "Modern Uses of Cultivated Algae". Ethnobotanical Leaflets. Southern Illinois University Carbondale. Archived from the original on 23 December 2008. Retrieved 26 December 2008.
- Mondragón, Jennifer; Mondragón, Jeff (2003). Seaweeds of the Pacific Coast. Monterey, California: Sea Challengers Publications. ISBN 978-0-930118-29-7.
- "Durvillaea antarctica (Chamisso) Hariot". AlgaeBase.
- "How marine algae could help feed the world". World Economic Forum. Retrieved 21 June 2018.
- "One solution to global hunger could be at the bottom of the ocean". World Economic Forum. Retrieved 21 June 2018.
- "Algae: Pond Scum or Food of the Future?". HowStuffWorks. 12 June 2018. Retrieved 21 June 2018.
- Rani, Komal; Sandal, Nidi; Sahoo, PK (2018). "A comprehensive review on chlorella- its composition, health benefits, market and regulatory scenario" (PDF). The Pharma Innovation Journal. 7 (7): 585. Archived (PDF) from the original on 9 October 2022. Retrieved 21 December 2020.
- Bantilan, C., MS, RD, CD. (2020, July 15). What's the difference between chlorella and spirulina? Healthline. https://www.healthline.com/nutrition/chlorella-spirulina
- Bigogno, C.; Khozin-Goldberg, I.; Boussiba, S.; Vonshak, A.; Cohen, Z. (2002). "Lipid and fatty acid composition of the green oleaginous alga Parietochloris incisa, the richest plant source of arachidonic acid". Phytochemistry. 60 (5): 497–503. doi:10.1016/S0031-9422(02)00100-0. PMID 12052516. Archived from the original on 1 October 2017.
- Aubrey, Allison (1 November 2007). "Getting Brain Food Straight from the Source". Morning Edition. NPR. Archived from the original on 3 November 2007.
- "Re-imagining algae". Australian Broadcasting Corporation. 12 October 2016. Archived from the original on 2 February 2017. Retrieved 26 January 2017.
- Morrissey, J.; Jones, M. S.; Harriott, V. (1988). "Nutrient cycling in the Great Barrier Reef Aquarium – Proceedings of the 6th International Coral Reef Symposium, Australia". ReefBase. Archived from the original on 23 February 2015.
- "Patent US4333263 – Algal turf scrubber". Archived from the original on 6 September 2011 – via Google Patent Search.
- "Hydromentia Water Treatment Technologies" (PDF). Archived from the original (PDF) on 24 September 2015.
- Veraart, Annelies J.; Romaní, Anna M.; Tornés, Elisabet; Sabater, Sergi (2008). "Algal Response to Nutrient Enrichment in Forested Oligotrophic Stream". Journal of Phycology. 44 (3): 564–572. doi:10.1111/j.1529-8817.2008.00503.x. PMID 27041416. S2CID 2040067. Archived from the original on 1 October 2010.
- "Algae: A Mean, Green Cleaning Machine". USDA Agricultural Research Service. 7 May 2010. Archived from the original on 19 October 2010.
- "Algae Biopolymers, Companies, Production, Market – Oilgae – Oil from Algae". oilgae.com. Retrieved 18 November 2017.
- "Renewable flip flops: scientists produce the 'No. 1' footwear in the world from algae". ZME Science. 9 October 2017. Retrieved 18 November 2017.
- "World's First Algae Surfboard Makes Waves in San Diego". Energy.gov. Retrieved 18 November 2017.
- Cappitelli, Francesca; Sorlini, Claudia (2008). "Microorganisms Attack Synthetic Polymers in Items Representing Our Cultural Heritage". Applied and Environmental Microbiology. 74 (3): 564–569. Bibcode:2008ApEnM..74..564C. doi:10.1128/AEM.01768-07. PMC 2227722. PMID 18065627.
- Arad, Shoshana; Spharim, Ishai (1998). "Production of Valuable Products from Microalgae: An Emerging Agroindustry". In Altman, Arie (ed.). Agricultural Biotechnology. Books in Soils, Plants, and the Environment. Vol. 61. CRC Press. p. 638. ISBN 978-0-8247-9439-2.
- Rathbun, C.; Doyle, A.; Waterhouse, T. (June 1994). "Measurement of Algal Chlorophylls and Carotenoids by HPLC" (PDF). Joint Global Ocean Flux Study Protocols. 13: 91–96. Archived from the original (PDF) on 4 March 2016. Retrieved 7 July 2014.
- Latasa, M.; Bidigare, R. (1998). "A comparison of phytoplankton populations of the Arabian Sea during the Spring Intermonsoon and Southwest Monsoon of 1995 as described by HPLC-analyzed pigments". Deep-Sea Research Part II. 45 (10–11): 2133–2170. Bibcode:1998DSRII..45.2133L. doi:10.1016/S0967-0645(98)00066-6.
Bibliography
General
- Chapman, V.J. (1950). Seaweeds and their Uses. London: Methuen. ISBN 978-0-412-15740-0.
- Fritsch, F. E. (1945) [1935]. The Structure and Reproduction of the Algae. Vol. I & II. Cambridge University Press.
- van den Hoek, C.; Mann, D. G.; Jahns, H. M. (1995). Algae: An Introduction to Phycology. Cambridge University Press.
- Kassinger, Ruth (2020). Slime: How Algae Created Us, Plague Us, and Just Might Save Us. Mariner.
- Lembi, C. A.; Waaland, J.R. (1988). Algae and Human Affairs. Cambridge University Press. ISBN 978-0-521-32115-0.
- Mumford, T. F.; Miura, A. (1988). "Porphyra as food: cultivation and economic". In Lembi, C. A.; Waaland, J. R. (eds.). Algae and Human Affairs. Cambridge University Press. pp. 87–117. ISBN 978-0-521-32115-0..
- Round, F. E. (1981). The Ecology of Algae. London: Cambridge University Press. ISBN 978-0-521-22583-0.
- Smith, G. M. (1938). Cryptogamic Botany. Vol. I. New York: McGraw-Hill.
- Ask, E.I (1990). Cottonii and Spinosum Cultivation Handbook. FMC BioPolymer Corporation.Philippines.
Regional
Britain and Ireland
- Brodie, Juliet; Burrows, Elsie M.; Chamberlain, Yvonne M.; Christensen, Tyge; Dixon, Peter Stanley; Fletcher, R. L.; Hommersand, Max H.; Irvine, Linda M.; Maggs, Christine A. (1977–2003). Seaweeds of the British Isles: A Collaborative Project of the British Phycological Society and the British Museum (Natural History). London / Andover: British Museum of Natural History, HMSO / Intercept. ISBN 978-0-565-00781-2.
- Cullinane, John P. (1973). Phycology of the South Coast of Ireland. Cork: Cork University Press.
- Hardy, F. G.; Aspinall, R. J. (1988). An Atlas of the Seaweeds of Northumberland and Durham. The Hancock Museum, University Newcastle upon Tyne: Northumberland Biological Records Centre. ISBN 978-0-9509680-5-6.
- Hardy, F. G.; Guiry, Michael D.; Arnold, Henry R. (2006). A Check-list and Atlas of the Seaweeds of Britain and Ireland (Revised ed.). London: British Phycological Society. ISBN 978-3-906166-35-3.
- John, D. M.; Whitton, B. A.; Brook, J. A. (2002). The Freshwater Algal Flora of the British Isles. Cambridge / New York: Cambridge University Press. ISBN 978-0-521-77051-4.
- Knight, Margery; Parke, Mary W. (1931). Manx Algae: An Algal Survey of the South End of the Isle of Man. Liverpool Marine Biology Committee Memoirs on Typical British Marine Plants & Animals. Vol. XXX. Liverpool: University Press.
- Morton, Osborne (1994). Marine Algae of Northern Ireland. Belfast: Ulster Museum. ISBN 978-0-900761-28-7.
- Morton, Osborne (1 December 2003). "The Marine Macroalgae of County Donegal, Ireland". Bulletin of the Irish Biogeographical Society. 27: 3–164.
Australia
- Huisman, J. M. (2000). Marine Plants of Australia. University of Western Australia Press. ISBN 978-1-876268-33-6.
New Zealand
- Chapman, Valentine Jackson; Lindauer, VW; Aiken, M.; Dromgoole, F. I. (1970) [1900, 1956, 1961, 1969]. The Marine algae of New Zealand. London / Lehre, Germany: Linnaean Society of London / Cramer.
Europe
- Cabioc'h, Jacqueline; Floc'h, Jean-Yves; Le Toquin, Alain; Boudouresque, Charles-François; Meinesz, Alexandre; Verlaque, Marc (1992). Guide des algues des mers d'Europe: Manche/Atlantique-Méditerranée (in French). Lausanne, Suisse: Delachaux et Niestlé. ISBN 978-2-603-00848-5.
- Gayral, Paulette (1966). Les Algues de côtes françaises (manche et atlantique), notions fondamentales sur l'écologie, la biologie et la systématique des algues marines (in French). Paris: Doin, Deren et Cie.
- Guiry, Michael. D.; Blunden, G. (1991). Seaweed Resources in Europe: Uses and Potential. John Wiley & Sons. ISBN 978-0-471-92947-5.
- Míguez Rodríguez, Luís (1998). Algas mariñas de Galicia: Bioloxía, gastronomía, industria (in Galician). Vigo: Edicións Xerais de Galicia. ISBN 978-84-8302-263-4.
- Otero, J. (2002). Guía das macroalgas de Galicia (in Galician). A Coruña: Baía Edicións. ISBN 978-84-89803-22-0.
- Bárbara, I.; Cremades, J. (1993). Guía de las algas del litoral gallego (in Spanish). A Coruña: Concello da Coruña – Casa das Ciencias.
Arctic
- Kjellman, Frans Reinhold (1883). The algae of the Arctic Sea: A survey of the species, together with an exposition of the general characters and the development of the flora. Vol. 20. Stockholm: Kungl. Svenska vetenskapsakademiens handlingar. pp. 1–350.
Greenland
- Lund, Søren Jensen (1959). The Marine Algae of East Greenland. Kövenhavn: C.A. Reitzel. 9584734.
Faroe Islands
- Børgesen, Frederik (1970) [1903]. "Marine Algae". In Warming, Eugene (ed.). Botany of the Faröes Based Upon Danish Investigations, Part II. Copenhagen: Det nordiske Forlag. pp. 339–532..
Canary Islands
- Børgesen, Frederik (1936) [1925, 1926, 1927, 1929, 1930]. Marine Algae from the Canary Islands. Copenhagen: Bianco Lunos.
Morocco
- Gayral, Paulette (1958). Algues de la côte atlantique marocaine (in French). Casablanca: Rabat [Société des sciences naturelles et physiques du Maroc].
South Africa
- Stegenga, H.; Bolton, J. J.; Anderson, R. J. (1997). Seaweeds of the South African West Coast. Bolus Herbarium, University of Cape Town. ISBN 978-0-7992-1793-3.
North America
- Abbott, I. A.; Hollenberg, G. J. (1976). Marine Algae of California. California: Stanford University Press. ISBN 978-0-8047-0867-8.
- Greeson, Phillip E. (1982). An annotated key to the identification of commonly occurring and dominant genera of Algae observed in the Phytoplankton of the United States. Washington DC: US Department of the Interior, Geological Survey. Retrieved 19 December 2008.
- Taylor, William Randolph (1969) [1937, 1957, 1962]. Marine Algae of the Northeastern Coast of North America. Ann Arbor: University of Michigan Press. ISBN 978-0-472-04904-2.
- Wehr, J. D.; Sheath, R. G. (2003). Freshwater Algae of North America: Ecology and Classification. Academic Press. ISBN 978-0-12-741550-5.
External links
- Guiry, Michael; Guiry, Wendy. "AlgaeBase". – a database of all algal names including images, nomenclature, taxonomy, distribution, bibliography, uses, extracts
- "Algae – Cell Centered Database". CCDb.UCSD.edu. San Diego: University of California.
- "Algae Research". National Museum of Natural History, Department of Botany. 2008. Archived from the original on 1 December 2008. Retrieved 19 December 2008.
- Anderson, Don; Keafer, Bruce; Kleindinst, Judy; Shaughnessy, Katie; Joyce, Katherine; Fino, Danielle; Shepherd, Adam (2007). "Harmful Algae". US National Office for Harmful Algal Blooms. Archived from the original on 5 December 2008. Retrieved 19 December 2008.
- "Australian Freshwater Algae (AFA)". Department of Environment / Climate Change NSW Botanic Gardens Trust. Archived from the original on 30 December 2008. Retrieved 19 December 2008.
- "Freshwater Algae Research". Phycology Section, Patrick Center for Environmental Research. 2011. Retrieved 17 December 2011.
- "Monterey Bay Flora". Monterey Bay Aquarium Research Institute (MBARI). 1996–2008. Archived from the original on 25 January 2009. Retrieved 20 December 2008.
- Silva, Paul (1997–2004). "Index Nominum Algarum (INA)". Berkeley: University Herbarium, University of California. Archived from the original on 23 December 2008. Retrieved 19 December 2008.
- "Algae: Protists with Chloroplasts". TolWeb.org.
- "Research on microalgae". Algae.WUR.nl. Wageningen UR. 2009. Archived from the original on 24 April 2009. Retrieved 18 May 2009.
- "Algae glossary". Australian Biological Resources Study. Archived from the original on 1 November 2012 – via Environment.gov.au.
- "About Algae". NMH.ac.uk. Natural History Museum, United Kingdom.
- EnAlgae Archived 4 September 2014 at the Wayback Machine