Cryptomonad
The cryptomonads (or cryptophytes)[1] are a group of algae,[2] most of which have plastids. They are common in freshwater, and also occur in marine and brackish habitats. Each cell is around 10–50 μm in size and flattened in shape, with an anterior groove or pocket. At the edge of the pocket there are typically two slightly unequal flagella.
| Cryptomonads | |
|---|---|
 | |
| Rhodomonas salina | |
| Scientific classification (incertae sedis within Eukaryota) | |
| Domain: | Eukaryota |
| (unranked): | |
| (unranked): | |
| Phylum: | Cryptophyta Cavalier-Smith, 1986 |
| Classes & Orders | |
| Synonyms | |
| |
Some may exhibit mixotrophy.[3]
Characteristics
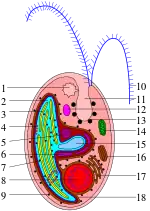
Cryptomonads are distinguished by the presence of characteristic extrusomes called ejectosomes, which consist of two connected spiral ribbons held under tension.[4] If the cells are irritated either by mechanical, chemical or light stress, they discharge, propelling the cell in a zig-zag course away from the disturbance. Large ejectosomes, visible under the light microscope, are associated with the pocket; smaller ones occur underneath the periplast, the cryptophyte-specific cell surrounding.[5][6]
Except for the class Goniomonadea, which lacks plastids entirely,[7] and Cryptomonas paramecium (previously called Chilomonas paramecium), which has leucoplasts, cryptomonads have one or two chloroplasts. These contain chlorophylls a and c, together with phycobiliproteins and other pigments, and vary in color (brown, red to blueish-green). Each is surrounded by four membranes, and there is a reduced cell nucleus called a nucleomorph between the middle two. This indicates that the plastid was derived from a eukaryotic symbiont, shown by genetic studies to have been a red alga.[8] However, the plastids are very different from red algal plastids: phycobiliproteins are present but only in the thylakoid lumen and are present only as phycoerythrin or phycocyanin. In the case of Rhodomonas, the crystal structure has been determined to 1.63 Å;[9] and it has been shown that the alpha subunit bears no relation to any other known phycobiliprotein.
A few cryptomonads, such as Cryptomonas, can form palmelloid stages, but readily escape the surrounding mucus to become free-living flagellates again. Some Cryptomonas species may also form immotile microbial cysts—resting stages with rigid cell walls to survive unfavorable conditions. Cryptomonad flagella are inserted parallel to one another, and are covered by bipartite hairs called mastigonemes, formed within the endoplasmic reticulum and transported to the cell surface. Small scales may also be present on the flagella and cell body. The mitochondria have flat cristae, and mitosis is open; sexual reproduction has also been reported.
Classification

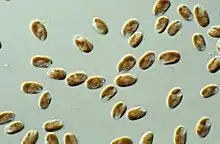
The first mention of cryptomonads appears to have been made by Christian Gottfried Ehrenberg in 1831,[10] while studying Infusoria. Later, botanists treated them as a separate algae group, class Cryptophyceae or division Cryptophyta, while zoologists treated them as the flagellate protozoa order Cryptomonadina. In some classifications, the cryptomonads were considered close relatives of the dinoflagellates because of their (seemingly) similar pigmentation, being grouped as the Pyrrhophyta. Cryptomonad chloroplasts are closely related to those of the heterokonts and haptophytes, and the three groups were united by Cavalier-Smith as the Chromista. However, the case that the organisms themselves are closely related was counter-indicated by the major differences in cell organization (ultrastructural identity), suggesting that the three major lineages assigned to the chromists had acquired plastids independently, and that chromists are polyphyletic. The perspective that cryptomonads are primitively heterotrophic and secondarily acquired chloroplasts, is supported by molecular evidence [11] Parfrey et al. and Burki et al. placed Cryptophyceae as a sister clade to the Green Algae.[12][13] The sister group to the cryptomonads is likely the kathablepharids (also referred to as katablepahrids), a group of flagellates that also have ejectisomes.[14]
One suggested grouping is as follows: (1) Cryptomonas, (2) Chroomonas/Komma and Hemiselmis, (3) Rhodomonas/Rhinomonas/Storeatula, (4) Guillardia/Hanusia, (5) Geminigera/Plagioselmis/Teleaulax, (6) Proteomonas sulcata, (7) Falcomonas daucoides.[15]
References
- Barnes, Richard Stephen Kent (2001). The Invertebrates: A Synthesis. Wiley-Blackwell. p. 41. ISBN 978-0-632-04761-1.
- Khan H, Archibald JM (May 2008). "Lateral transfer of introns in the cryptophyte plastid genome". Nucleic Acids Res. 36 (9): 3043–53. doi:10.1093/nar/gkn095. PMC 2396441. PMID 18397952.
- "Cryptophyta - the cryptomonads". Archived from the original on 2011-06-10. Retrieved 2009-06-02.
- Graham, L. E.; Graham, J. M.; Wilcox, L. W. (2009). Algae (2nd ed.). San Francisco, CA: Benjamin Cummings (Pearson). ISBN 9780321559654.
- Morrall, S.; Greenwood, A. D. (1980). "A comparison of the periodic sub-structures of the trichocysts of the Cryptophyceae and Prasinophyceae". BioSystems. 12 (1–2): 71–83. doi:10.1016/0303-2647(80)90039-8. PMID 6155157.
- Grim, J. N.; Staehelin, L. A. (1984). "The ejectisomes of the flagellate Chilomonas paramecium - Visualization by freeze-fracture and isolation techniques". Journal of Protozoology. 31 (2): 259–267. doi:10.1111/j.1550-7408.1984.tb02957.x. PMID 6470985.
- Nuclear genome sequence of the plastid-lacking cryptomonad Goniomonas avonlea provides insights into the evolution of secondary plastids
- Douglas, S.; et al. (2002). "The highly reduced genome of an enslaved algal nucleus". Nature. 410 (6832): 1091–1096. Bibcode:2001Natur.410.1091D. doi:10.1038/35074092. PMID 11323671.
- Wilk, K.; et al. (1999). "Evolution of a light-harvesting protein by addition of new subunits and rearrangement of conserved elements: Crystal structure of a cryptophyte phycoerythrin at 1.63Å resolution". PNAS. 96 (16): 8901–8906. doi:10.1073/pnas.96.16.8901. PMC 17705. PMID 10430868.
- Novarino, G. (2012). "Cryptomonad taxonomy in the 21st century: The first 200 years". Phycological Reports: Current Advances in Algal Taxonomy and Its Applications: Phylogenetic, Ecological and Applied Perspective: 19–52. Retrieved 2018-10-16.
- Cenci U, Sibbald SJ, Curtis BA, Kamikawa R, Eme L, Moog D, Henrissat B, Maréchal E, Chabi M, Djemiel C, Roger AJ, Kim E, Archibald JM. Nuclear genome sequence of the plastid-lacking cryptomonad Goniomonas avonlea provides insights into the evolution of secondary plastids. BMC Biol. 2018 Nov 28;16(1):137. doi: 10.1186/s12915-018-0593-5. PMID: 30482201; PMCID: PMC6260743.
- Laura Wegener Parfrey; Daniel J G Lahr; Andrew H Knoll; Laura A Katz (16 August 2011). "Estimating the timing of early eukaryotic diversification with multigene molecular clocks" (PDF). Proceedings of the National Academy of Sciences of the United States of America. 108 (33): 13624–9. Bibcode:2011PNAS..10813624P. doi:10.1073/PNAS.1110633108. ISSN 0027-8424. PMC 3158185. PMID 21810989. Wikidata Q24614721.
- Burki, Fabien; Kaplan, Maia; Tikhonenkov, Denis V.; Zlatogursky, Vasily; Minh, Bui Quang; Radaykina, Liudmila V.; Smirnov, Alexey; Mylnikov, Alexander P.; Keeling, Patrick J. (2016-01-27). "Untangling the early diversification of eukaryotes: a phylogenomic study of the evolutionary origins of Centrohelida, Haptophyta and Cryptista". Proc. R. Soc. B. 283 (1823): 20152802. doi:10.1098/rspb.2015.2802. ISSN 0962-8452. PMC 4795036. PMID 26817772.
- Yuki, N., Keitaro, K., Keito, S., Goro, T., Takashi, S., Ken-ichiro, I., Tetsuo, H., Yuji, I. and Moriya. 2020. Mitochondrial Genomes of Hemiarma marina and Leucocryptos marina Revised the Evolution of Cytochrome c Maturation in Cryptista Frontiers in Ecology and Evolution: 8, DOI=10.3389/fevo.2020.00140
- "Cryptomonads". Retrieved 2009-06-24.