Bempedoic acid
Bempedoic acid, sold under the brand name Nexletol among others, is a medication for the treatment of hypercholesterolemia (high blood cholesterol levels).[2][3]
| Clinical data | |
|---|---|
| Trade names | Nexletol, Nilemdo |
| Other names | ESP-55016, ETC-1002 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a620020 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 99.3%[2] |
| Metabolism | Glucuronidation |
| Elimination half-life | 21±11 hrs |
| Excretion | 70% urine, 30% feces |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.238.679 |
| Chemical and physical data | |
| Formula | C19H36O5 |
| Molar mass | 344.492 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
The most common side effects include hyperuricemia (high blood levels of uric acid), pain in arms or legs, and anemia (low red blood cell counts).[3]
Bempedoic acid blocks an enzyme in the liver called adenosine triphosphate-citrate lyase, which is involved in making cholesterol.[3]
Bempedoic acid was approved for use in the United States in February 2020, and for use in the European Union in April 2020.[3][4][5] The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication.[6]
Medical uses
In the US, bempedoic acid is indicated for the treatment of hypercholesterolemia in combination with diet and the highest tolerated statin therapy in adults with heterozygous familial hypercholesterolemia, or with established atherosclerotic cardiovascular disease, who need additional lowering of LDL cholesterol.[2]
In the EU, bempedoic acid is indicated in adults with primary hypercholesterolaemia (heterozygous familial and non familial) or mixed dyslipidaemia, as an adjunct to diet in combination with a statin or statin with other lipid-lowering therapies in patients unable to reach LDL-C goals with the maximum tolerated dose of a statin; or alone or in combination with other lipid-lowering therapies in patients who are statin intolerant, or for whom a statin is contraindicated.[3]
Side effects
Common adverse effects in clinical trials were muscle spasms (3.6% of treated patients, as compared to 2.3% under placebo), pain in the back (3.3% versus 2.2%) or in a limb (3.0% versus 1.7%), gout (1.5% versus 0.4%), and gastrointestinal problems such as diarrhea. A less common but more serious adverse effect was tendon rupture in the rotator cuff of the shoulder, the biceps tendon or the Achilles tendon (0.5% versus 0.0%).[2]
Interactions
Bempedoic acid does not interact with the cytochrome P450 enzyme system in the liver and only weakly inhibits the transporter proteins SLCO1B1, SLCO1B3 and SLC22A7 (the latter possibly being responsible for the increase of uric acid in the blood, and therefore the adverse effect gout). Despite this, the drug increases blood levels of statins. The effect is most pronounced with simvastatin and pravastatin, whose AUC is increased about twofold. No other clinically relevant interactions have been found in studies.[2]
Pharmacology
Mechanism of action
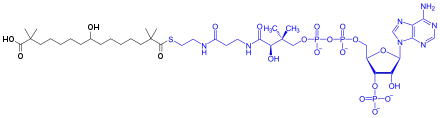
Bempedoic acid is a prodrug. It is activated to the thioester with coenzyme A by the enzyme SLC27A2 in the liver.[7] The activated substance inhibits ATP citrate lyase, which is involved in the liver's biosynthesis of cholesterol upstream of HMG-CoA reductase, the enzyme that is blocked by statins.[8][9]
The substance also activates AMP-activated protein kinase, but this effect is likely not relevant in humans.[7]
Pharmacokinetics
Following oral intake, bempedoic acid reaches highest blood plasma concentrations after 3.5 hours.[2] Food does not affect its absorption.[2] When in the bloodstream, 99.3% of the substance are bound to plasma proteins.[2] About a fifth of the substance is reversibly converted by an aldo-keto reductase enzyme to a metabolite (called ESP15228) that is also pharmacologically active in form of its coenzyme A–thioester.[2] Of ESP15228, 99.2% are bound to plasma proteins.[2] Both bempedoic acid and the metabolite are inactivated by glucuronidation of their carboxylic acid groups.[2]
Bempedoic acid has a biological half-life of 21±11 hours.[2] Over 95% of the substance are excreted in form of metabolites; about 70% with the urine and 30% with the feces.[2]
History
There were two clinical trials that evaluated the benefits and side effects of bempedoic acid.[5] The trial designs were similar.[5] All enrolled subjects were on a lipid-lowering diet and taking the highest dose of a statin (drug commonly used to lower cholesterol) for high cholesterol.[5] In both trials, subjects were randomly assigned to receive bempedoic acid or placebo tablets every day for 52-weeks.[5] Neither the subjects nor the health care providers knew which treatment was being given.[5] The trials measured percent change in LDL cholesterol (LDL-C) blood levels from baseline to week twelve and compared bempedoic acid to placebo.[5] In one clinical trial, bempedoic acid reduced LDL-C by about 20 mg/dl compared to placebo and had a similar frequency of side effects to placebo, although a higher percentage of drug receiving subjects dropped out of the study because of side effects (11% vs. 7% under placebo).[8] As of March 2019, its effects on cardiovascular morbidity and mortality have not been determined; but studies are under way.[9]
In January 2020, the Committee for Medicinal Products for Human Use (CHMP) in the European Union recommended granting of a marketing authorization for bempedoic acid as both a standalone drug (brand name Nilemdo)[10] and as a fixed-dose combination medication with ezetimibe (brand name Nustendi).[11] Bempedoic acid was approved for use in the European Union in April 2020,[3] and the combination bempedoic acid/ezetimibe was approved in March 2020.[12]
In February 2020, bempedoic acid was approved for use in the United States both as a standalone drug (brand name Nexetol)[4][13][14][15] The U.S. Food and Drug Administration (FDA) granted the approval of Nexletol to Esperion Therapeutics.[2][4] and in a fixed-dose combination with ezetimibe (brand name Nexlizet).[16]
The FDA approved bempedoic acid based on evidence from two clinical trials (Trial 1/ NCT02666664 and Trial 2/NCT02991118) of 3009 subjects with high LDL cholesterol and known atherosclerotic cardiovascular disease or HeFH.[5] The trials were conducted in United States, Canada, and Europe.[5]
See also
References
- "Nilemdo 180mg film-coated tablets - Summary of Product Characteristics (SmPC)". (emc). 4 September 2020. Retrieved 17 January 2021.
- "Nexletol- bempedoic acid tablet, film coated". DailyMed. 10 March 2020. Retrieved 19 March 2020.
- "Nilemdo EPAR". European Medicines Agency (EMA). 29 January 2020. Retrieved 24 April 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- "Drug Approval Package: Nexletol". U.S. Food and Drug Administration (FDA). 24 March 2020. Retrieved 17 January 2021.
- "Drug Trials Snapshots: Nexletol". U.S. Food and Drug Administration. 21 February 2020. Retrieved 27 March 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "New Drug Therapy Approvals 2020". U.S. Food and Drug Administration (FDA). 31 December 2020. Retrieved 17 January 2021.
- Bilen O, Ballantyne CM (October 2016). "Bempedoic Acid (ETC-1002): An Investigational Inhibitor of ATP Citrate Lyase". Current Atherosclerosis Reports. 18 (10): 61. doi:10.1007/s11883-016-0611-4. PMC 5035316. PMID 27663902.
- Ray KK, Bays HE, Catapano AL, et al. (CLEAR Harmony Trial) (March 2019). "Safety and Efficacy of Bempedoic Acid to Reduce LDL Cholesterol". New England Journal of Medicine. 380 (11): 1022–32. doi:10.1056/NEJMoa1803917. PMID 30865796.
- "Bempedoic Acid". Esperion Therapeutics. Retrieved 15 March 2019.
- "Nilemdo: Pending EC decision". European Medicines Agency (EMA). 30 January 2020. Retrieved 21 February 2020.
- "Nustendi: Pending EC decision". European Medicines Agency (EMA). 30 January 2020. Retrieved 21 February 2020.
- "Nustendi EPAR". European Medicines Agency (EMA). 29 January 2020. Retrieved 17 January 2021.
- "Esperion Announces FDA Approval of Nexletol (bempedoic acid) Tablet, an Oral, Once-Daily, Non-Statin LDL-Cholesterol Lowering Medicine". Esperion Therapeutics, Inc. (Press release). 21 February 2020. Retrieved 21 February 2020.
- "FDA Approves Drug That Lowers Cholesterol in a New Way". The New York Times. Associated Press. 21 February 2020. Retrieved 21 February 2020.
- McGinley L (21 February 2020). "FDA approves first non-statin pill to treat high cholesterol in almost two decades". The Washington Post. Retrieved 21 February 2020.
- "Drug Approval Package: NEXLIZET". U.S. Food and Drug Administration (FDA). 17 April 2020. Retrieved 10 July 2021.
Further reading
- Saeed A, Ballantyne CM (May 2018). "Bempedoic Acid (ETC-1002): A Current Review". Cardiol Clin. 36 (2): 257–264. doi:10.1016/j.ccl.2017.12.007. PMID 29609755.
- Zagelbaum NK, Yandrapalli S, Nabors C, Frishman WH (2019). "Bempedoic Acid (ETC-1002): ATP Citrate Lyase Inhibitor: Review of a First-in-Class Medication with Potential Benefit in Statin-Refractory Cases". Cardiol Rev. 27 (1): 49–56. doi:10.1097/CRD.0000000000000218. PMID 29939848. S2CID 49411718.
External links
- "Bempedoic acid". Drug Information Portal. U.S. National Library of Medicine.
- Clinical trial number NCT02666664 for "Evaluation of Long-Term Safety and Tolerability of ETC-1002 in High-Risk Patients With Hyperlipidemia and High CV Risk (CLEAR Harmony)" at ClinicalTrials.gov
- Clinical trial number NCT02988115 for "Evaluation of the Efficacy and Safety of Bempedoic Acid (ETC-1002) in Patients With Hyperlipidemia and Statin Intolerant (CLEAR Serenity)" at ClinicalTrials.gov
- Clinical trial number NCT02991118 for "Evaluation of Long-Term Efficacy of Bempedoic Acid (ETC-1002) in Patients With Hyperlipidemia at High Cardiovascular Risk (CLEAR Wisdom)" at ClinicalTrials.gov