Probucol
Probucol, sold under the trade name Lorelco among others, is an anti-hyperlipidemic drug[1] initially developed for the treatment of coronary artery disease. Clinical development was discontinued after it was found that the drug may have the undesired effect of lowering HDL in patients with a previous history of heart disease. It may also cause QT interval prolongation.
 | |
| Clinical data | |
|---|---|
| Trade names | Lorelco |
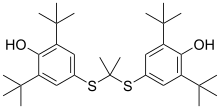
| Other names | 2,6-di-tert-butyl-4-({2-[(3,5-di-tert-butyl-4-hydroxyphenyl)sulfanyl]propan-2-yl}sulfanyl)phenol |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a611037 |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.041.404 |
| Chemical and physical data | |
| Formula | C31H48O2S2 |
| Molar mass | 516.84 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Probucol was initially developed in the 1970s by a chemical company to maximize airplane tire longevity.
Mechanism of action
Probucol lowers the level of cholesterol in the bloodstream by increasing the rate of LDL catabolism. Additionally, probucol may inhibit cholesterol synthesis and delay cholesterol absorption.[2] Probucol is a powerful antioxidant which inhibits the oxidation of cholesterol in LDLs; this slows the formation of foam cells, which form atherosclerotic plaques. After promising test results in mouse models, Probucol is under study at Weston Brain Institute of McGill University as a possible aid in delaying the onset of Alzheimer's disease.
Probucol has also been shown to inhibit ABCA1-dependent cholesterol transport,[3] which may contribute to its known effect of lowering HDL.[4]
References
- Yamamoto A (11 December 2008). "A Unique Antilipidemic Drug - Probucol". J. Atheroscler. Thromb. 15 (6): 304–5. doi:10.5551/jat.E621. PMID 19075491. Retrieved 2020-01-29.
- "Probucol (Systemic)". Drugs.com. 1998-08-24. Retrieved 2020-01-29.
- Favari E, Zanotti I, Zimetti F, Ronda N, Bernini F, Rothblat GH (28 October 2004). "Probucol inhibits ABCA1-mediated cellular lipid efflux". Arterioscler. Thromb. Vasc. Biol. 24 (12): 2345–50. doi:10.1161/01.ATV.0000148706.15947.8a. PMID 15514211. Retrieved 2020-01-29.
- Miida T, Seino U, Miyazaki O, Hanyu O, Hirayama S, Saito T, Ishikawa Y, Akamatsu S, Nakano T, Katsuyuki N, Okazaki M, Okada M (October 2008). "Probucol markedly reduces HDL phospholipids and elevated prebeta1-HDL without delayed conversion into alpha-migrating HDL: putative role of angiopoietin-like protein 3 in probucol-induced HDL remodeling". Atherosclerosis. 200 (2): 329–35. doi:10.1016/j.atherosclerosis.2007.12.031. PMID 18279878. Retrieved 2020-01-29.