Chorion
The chorion is the outermost fetal membrane around the embryo in mammals, birds and reptiles (amniotes). It develops from an outer fold on the surface of the yolk sac, which lies outside the zona pellucida (in mammals), known as the vitelline membrane in other animals. In insects it is developed by the follicle cells while the egg is in the ovary.[1]
| Chorion | |
|---|---|
 Diagram showing the chorion of a chicken egg | |
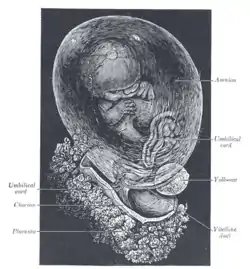 Human fetus, enclosed in the amnion | |
| Details | |
| Identifiers | |
| Latin | chorion |
| MeSH | D002823 |
| TE | E5.11.3.1.1.0.3 |
| Anatomical terminology | |
Structure
In humans and other mammals (excluding monotremes), the chorion is one of the fetal membranes that exist during pregnancy between the developing fetus and mother. The chorion and the amnion together form the amniotic sac. In humans it is formed by extraembryonic mesoderm and the two layers of trophoblast that surround the embryo and other membranes; the chorionic villi emerge from the chorion, invade the endometrium, and allow the transfer of nutrients from maternal blood to fetal blood.
Layers
The chorion consists of two layers: an outer formed by the trophoblast, and an inner formed by the somatic mesoderm.
The trophoblast is made up of an internal layer of cubical or prismatic cells, the cytotrophoblast or layer of Langhans, and an external multinucleated layer, the syncytiotrophoblast.
Growth
The chorion undergoes rapid proliferation and forms numerous processes, the chorionic villi, which invade and destroy the uterine decidua, while simultaneously absorbing nutritive materials from it for the growth of the embryo.
The chorionic villi are at first small and non-vascular, and consist of the trophoblast only, but they increase in size and ramify, whereas the mesoderm, carrying branches of the umbilical vessels, grows into them, and they are vascularized.
Blood is carried to the villi by the paired umbilical arteries, which branch into chorionic arteries and enter the chorionic villi as cotyledon arteries. After circulating through the capillaries of the villi, the blood is returned to the embryo by the umbilical vein. Until about the end of the second month of pregnancy, the villi cover the entire chorion, and are almost uniform in size; but, after this, they develop unequally.
Parts

The part of the chorion that is in contact with the decidua capsularis undergoes atrophy, so that by the fourth month scarcely a trace of the villi is left. This part of the chorion becomes smooth,[2] and is named the chorion laeve (from the Latin word levis, meaning smooth). As it takes no share in the formation of the placenta, this is also named the non-placental part of the chorion. As the chorion grows, the chorion laeve comes in contact with the decidua parietalis and these layers fuse.
The villi at the embryonic pole, which is in contact with the decidua basalis, increase greatly in size and complexity, and hence this part is named the chorion frondosum.[2]
Thus the placenta develops from the chorion frondosum and the decidua basalis.
Monochorionic twins
Monochorionic twins are twins that share the same placenta. This occurs in 0.3% of all pregnancies,[3] and in 75% of monozygotic (identical) twins, when the split takes place on or after the third day after fertilization.[4] The remaining 25% of monozygous twins become dichorionic diamniotic.[4] The condition may affect any type of multiple birth, resulting in monochorionic multiples.
Infections
Recent studies indicate that the chorion may be susceptible to pathogenic infections.[5] Recent findings indicate that Ureaplasma parvum bacteria can infect the chorion tissue, thereby impacting pregnancy outcome.[6] In addition, footprints of JC polyomavirus and Merkel cell polyomavirus have been detected in chorionic villi from females affected by spontaneous abortion as well as pregnant women.[7][8] Another virus, BK polyomavirus has been detected in the same tissues, but with lesser extent.[9]
Other animals
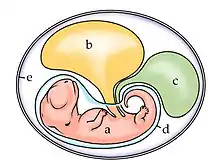
In reptiles, birds, and monotremes, the chorion is one of the four extraembryonic membranes that make up the amniotic egg that provide for the nutrients and protection needed for the embryo's survival. It is located inside the albumen, which is the white of the egg. It encloses the embryo and the rest of the embryonic system. The chorion is also present in insects. During growth and development of the embryo, there is an increased need for oxygen. To compensate for this, the chorion and the allantois fuse together to form the chorioallantoic membrane. Together these form a double membrane, which functions to remove carbon dioxide and to replenish oxygen through the porous shell. At the time of hatching, the fetus becomes detached from the chorion as it emerges from the shell.
Additional images
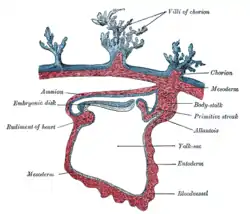 Section through the embryo.
Section through the embryo.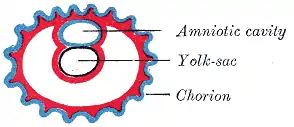 Diagram of the human embryo.
Diagram of the human embryo.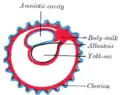 Diagram illustrating early formation of allantois and differentiation of body-stalk.
Diagram illustrating early formation of allantois and differentiation of body-stalk.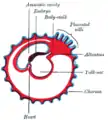 Diagram showing later stage of allantoic development with commencing constriction of the yolk-sac.
Diagram showing later stage of allantoic development with commencing constriction of the yolk-sac.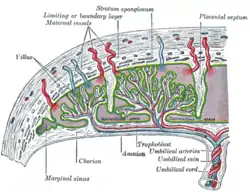 Scheme of placental circulation.
Scheme of placental circulation.
See also
- Choriogenesis
- Chorioamnionitis, an inflammation of the chorion and amnion, usually due to bacterial infection
- Chorionic hematoma
- Gestational trophoblastic disease, any abnormal proliferation of the trophoblasts, including choriocarcinoma, a highly invasive cancer.
References
![]() This article incorporates text in the public domain from page 60 of the 20th edition of Gray's Anatomy (1918)
This article incorporates text in the public domain from page 60 of the 20th edition of Gray's Anatomy (1918)
- Chapman, R.F. (1998) "The insects: structure and function", Section The egg and embryology. Previewed in Google Books on 26 Sep 2009.
- Genbačev, O; Vićovac, L; Larocque, N (July 2015). "The role of chorionic cytotrophoblasts in the smooth chorion fusion with parietal decidua". Placenta. 36 (7): 716–22. doi:10.1016/j.placenta.2015.05.002. PMC 4476638. PMID 26003500.
- Cordero L, Franco A, Joy SD, O'shaughnessy RW (December 2005). "Monochorionic diamniotic infants without twin-to-twin transfusion syndrome". Journal of Perinatology. 25 (12): 753–8. doi:10.1038/sj.jp.7211405. PMID 16281049.
- Shulman, Lee S.; van Vugt, John M. G. (2006). Prenatal medicine. Washington, DC: Taylor & Francis. p. 447. ISBN 0-8247-2844-0.
- Contini C, Rotondo JC, Magagnoli F, Maritati M, Seraceni S, Graziano A (2019). "Investigation on silent bacterial infections in specimens from pregnant women affected by spontaneous miscarriage". J Cell Physiol. 34 (3): 433–440. doi:10.1002/jcp.26952. PMID 30078192.
- Contini C, Rotondo JC, Magagnoli F, Maritati M, Seraceni S, Graziano A, Poggi A, Capucci R, Vesce F, Tognon M, Martini F (2018). "Investigation on silent bacterial infections in specimens from pregnant women affected by spontaneous miscarriage". J Cell Physiol. 234 (1): 100–9107. doi:10.1002/jcp.26952. PMID 30078192.
- Tagliapietra A, Rotondo JC, Bononi I, Mazzoni E, Magagnoli F, Maritati M (2019). "Footprints of BK and JC polyomaviruses in specimens from females affected by spontaneous abortion". Hum Reprod. 34 (3): 433–440. doi:10.1002/jcp.27490. hdl:11392/2397717. PMID 30590693. S2CID 53106591.
- Tagliapietra A, Rotondo JC, Bononi I, Mazzoni E, Magagnoli F, Maritati M (2020). "Droplet-digital PCR assay to detect Merkel cell polyomavirus sequences in chorionic villi from spontaneous abortion affected females". J Cell Physiol. 235 (3): 1888–1894. doi:10.1002/jcp.29213. PMID 31549405.
- Tagliapietra A, Rotondo JC, Bononi I, Mazzoni E, Magagnoli F, Maritati M (2019). "Footprints of BK and JC polyomaviruses in specimens from females affected by spontaneous abortion". Hum Reprod. 34 (3): 433–440. doi:10.1002/jcp.27490. hdl:11392/2397717. PMID 30590693. S2CID 53106591.
External links
- Histology image: 19903loa – Histology Learning System at Boston University — "Female Reproductive System: placenta, chorionic plate"
- McGill