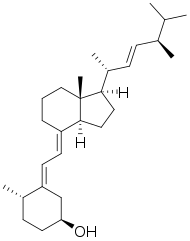
Dihydrotachysterol
Dihydrotachysterol (DHT) is a synthetic vitamin D analog activated in the liver that does not require renal hydroxylation like vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol). DHT has a rapid onset of action (2 hours), a shorter half-life, and a greater effect on mineralization of bone salts than does vitamin D.[1]
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a682335 |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.611 |
| Chemical and physical data | |
| Formula | C28H46O |
| Molar mass | 398.675 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
References
- Gagnon R, Ogden GW, Just G, Kaye M (April 1974). "Comparison of dihydrotachysterol and 5,6-trans vitamin D3 on intestinal calcium absorption in patients with chronic renal failure". Canadian Journal of Physiology and Pharmacology. 52 (2): 272–4. doi:10.1139/y74-037. PMID 4365509.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.