Fibrinopeptide
The fibrinopeptides, fibrinopeptide A (FpA) and fibrinopeptide B (FpB), are peptides which are located in the central region of the fibrous glycoprotein fibrinogen (factor I) and are cleaved by the enzyme thrombin (factor IIa) to convert fibrinogen into covalently-linked fibrin (factor IA) monomers.[1][2] The N-terminal FpA is cleaved from the Aα chains of fibrinogen and FpB from the Bβ chains of fibrinogen, with FpA released before FpB.[3][4] Subsequent to their formation, fibrin monomers are converted to cross-linked fibrin polymers by the action of thrombin-activated factor XIII (fibrin stabilizing factor), and these fibrin polymers form the backbone of a thrombus (blood clot).[2] Hence, the fibrinopeptides are sensitive markers of fibrinogenesis (fibrin generation), thrombin activity, and coagulation.[5][6][7][8]
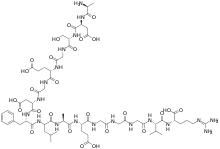 | |
| Names | |
|---|---|
| IUPAC name
(4S)-4-[[2-[[(2S)-2-[[(2S)-2-[[(2S)-2-aminopropanoyl]amino]-3-carboxypropanoyl]amino]-3-hydroxypropanoyl]amino]acetyl]amino]-5-[[2-[[(2S)-3-carboxy-1-[[(2S)-1-[[1-[[(2S)-1-[[(2S)-4-carboxy-1-[[2-[[2-[[2-[[(2S)-1-[[(1S)-1-carboxy-4-(diaminomethylideneamino)butyl]amino]-3-methyl-1-oxobutan-2-yl]amino]-2-oxoethyl]amino]-2-oxoethyl]amino]-2-oxoethyl]amino]-1-oxobutan-2-yl]amino]-1-oxopropan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-1-oxopropan-2-yl]amino]-2-oxoethyl]amino]-5-oxopentanoic acid | |
| Other names
Fibrinopeptide A; Fibrinopeptide A (human); FpA; FPA | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C63H97N19O26 |
| Molar mass | 1536.57 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
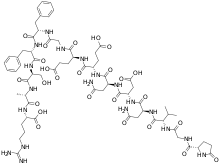 | |
| Names | |
|---|---|
| IUPAC name
(4S)-4-[[(2S)-2-[[(2S)-4-amino-2-[[(2S)-2-[[(2S)-4-amino-2-[[(2S)-3-methyl-2-[[2-[[(2S)-5-oxopyrrolidine-2-carbonyl]amino]acetyl]amino]butanoyl]amino]-4-oxobutanoyl]amino]-3-carboxypropanoyl]amino]-4-oxobutanoyl]amino]-4-carboxybutanoyl]amino]-5-[[2-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(1S)-4-carbamimidamido-1-carboxybutyl]amino]-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-2-oxoethyl]amino]-5-oxopentanoic acid | |
| Other names
Fibrinopeptide B; Fibrinopeptide B (human); FpB; FPB | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C66H93N19O25 |
| Molar mass | 1552.569 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
FpA is a 16-amino acid peptide.[8] The half-life of FpA is very short at approximately 3 to 5 minutes.[5][8] Hence, FpA levels provide a relatively transient measure of coagulation activation.[8]
Levels of FpA increase with age.[5] FpA levels also gradually increase throughout pregnancy.[9][10] Likewise, FpA levels have been reported to increase with estrogen therapy, including with combined birth control pills and menopausal hormone therapy, although research on FpA levels with these therapies appears to be relatively limited.[11][12][13][7]
References
- Weisel JW (2005). "Fibrinogen and fibrin". Adv Protein Chem. Advances in Protein Chemistry. 70: 247–99. doi:10.1016/S0065-3233(05)70008-5. ISBN 9780120342709. PMID 15837518.
- Gentry PA (November 2004). "Comparative aspects of blood coagulation". Vet J. 168 (3): 238–51. doi:10.1016/j.tvjl.2003.09.013. PMID 15501141.
- Wolberg AS (September 2012). "Determinants of fibrin formation, structure, and function". Curr Opin Hematol. 19 (5): 349–56. doi:10.1097/MOH.0b013e32835673c2. PMID 22759629. S2CID 11358104.
- O'Riordan, Máiread N; Higgins, John R (June 2003). "Haemostasis in normal and abnormal pregnancy". Best Practice & Research Clinical Obstetrics & Gynaecology. 17 (3): 385–396. doi:10.1016/S1521-6934(03)00019-1. ISSN 1521-6934. PMID 12787533.
- Mannucci PM (October 1994). "Mechanisms, markers and management of coagulation activation". Br Med Bull. 50 (4): 851–70. doi:10.1093/oxfordjournals.bmb.a072930. PMID 7804735.
- Vincent Marks; Thomas Cantor; Dusan Mesko; Rudolf Pullmann; Gabriela Nosalova (6 December 2012). Differential Diagnosis by Laboratory Medicine: A Quick Reference for Physicians. Springer Science & Business Media. pp. 443–. ISBN 978-3-642-55600-5. OCLC 1262382180.
- Farris M, Bastianelli C, Rosato E, Brosens I, Benagiano G (October 2017). "Pharmacodynamics of combined estrogen-progestin oral contraceptives: 2. effects on hemostasis". Expert Rev Clin Pharmacol. 10 (10): 1129–1144. doi:10.1080/17512433.2017.1356718. PMID 28712325. S2CID 205931204.
- Merlini PA, Ardissino D (1995). "Laboratory Measurement of Thrombin Activity--What Every Clinician Scientist Needs to Know". J Thromb Thrombolysis. 2 (2): 85–92. doi:10.1007/BF01064374. PMID 10608009. S2CID 28203940.
- Hellgren M (April 2003). "Hemostasis during normal pregnancy and puerperium". Semin Thromb Hemost. 29 (2): 125–30. doi:10.1055/s-2003-38897. PMID 12709915.
- Koltsova, Ekaterina; Balandina, Anna; Serebriyskiy, Ilya; Vuimo, Tatiana; Panteleev, Mikhail; Ataullakhanov, Fazoil (21 September 2016). "Classic and Global Hemostasis Testing in Pregnancy and during Pregnancy Complications". Seminars in Thrombosis and Hemostasis. 42 (7): 696–716. doi:10.1055/s-0036-1592303. eISSN 1098-9064. ISSN 0094-6176. PMID 27652600.
- Douxfils J, Morimont L, Bouvy C (November 2020). "Oral Contraceptives and Venous Thromboembolism: Focus on Testing that May Enable Prediction and Assessment of the Risk". Semin Thromb Hemost. 46 (8): 872–886. doi:10.1055/s-0040-1714140. PMID 33080636. S2CID 224821517.
- Canonico M (July 2014). "Hormone therapy and hemostasis among postmenopausal women: a review" (PDF). Menopause. 21 (7): 753–62. doi:10.1097/GME.0000000000000296. PMID 24937030. S2CID 20851353.
- Baker L, Meldrum KK, Wang M, Sankula R, Vanam R, Raiesdana A, Tsai B, Hile K, Brown JW, Meldrum DR (December 2003). "The role of estrogen in cardiovascular disease". J Surg Res. 115 (2): 325–44. doi:10.1016/s0022-4804(03)00215-4. PMID 14697301.