Hachimycin
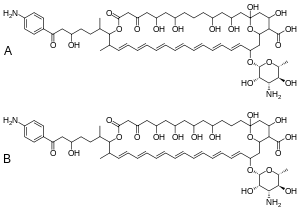
Hachimycin, also known as trichomycin,[1] is a polyene macrolide antibiotic,[2] antiprotozoal,[3] and antifungal derived from streptomyces.[4] It was first described in 1950, and in most research cases have been used for gynecological infections.[3]
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C116H168N4O36 |
| Molar mass | 2194.612 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
References
- "Trichomycin - C116H168N4O36 - PubChem". Retrieved July 7, 2016.
- Mechlinski W, Schaffner CP (June 1980). "Characterization of aromatic heptaene macrolide antibiotics by high performance liquid chromatography". The Journal of Antibiotics. 33 (6): 591–9. doi:10.7164/antibiotics.33.591. PMID 6893446.
- Nakano, Hiroshi; Hattori, Kiyoshi; Seki, Masahiro; Hirata, Yoshimasa (1956). "Studies on Trichomycin. III". The Journal of Antibiotics, Series A. 9 (5): 172–175. doi:10.11554/antibioticsa.9.5_172.
- Komori T (July 1990). "Trichomycin B, a polyene macrolide from Streptomyces". The Journal of Antibiotics. 43 (7): 778–82. doi:10.7164/antibiotics.43.778. PMID 2387771.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.