Marine microbial symbiosis
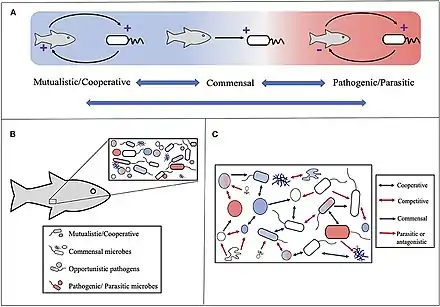
Microbial symbiosis in marine animals was not discovered until 1981.[3] In the time following, symbiotic relationships between marine invertebrates and chemoautotrophic bacteria have been found in a variety of ecosystems, ranging from shallow coastal waters to deep-sea hydrothermal vents. Symbiosis is a way for marine organisms to find creative ways to survive in a very dynamic environment. They are different in relation to how dependent the organisms are on each other or how they are associated. It is also considered a selective force behind evolution in some scientific aspects. The symbiotic relationships of organisms has the ability to change behavior, morphology and metabolic pathways. With increased recognition and research, new terminology also arises, such as holobiont, which the relationship between a host and its symbionts as one grouping.[4] Many scientists will look at the hologenome, which is the combined genetic information of the host and its symbionts. These terms are more commonly used to describe microbial symbionts.
| Part of a series on |
| Microbiomes |
|---|
 |
|
The type of marine animal vary greatly, for example, sponges, sea squirts, corals, worms, and algae all host a variety of unique symbionts.[5] Each symbiotic relationship displays a unique ecological niche, which in turn can lead to entirely new species of host species and symbiont.[3]
It is particularly interesting that it took so long to discover the marine microbial symbiosis because nearly every surface submerged in the oceans becomes covered with biofilm,[6] including a large number of living organisms. Many marine organisms display symbiotic relationships with microbes. Epibiotic bacteria have been found to live on crustacean larvae and protect them from fungal infections.[6] Other microbes in deep-sea vents have been found to prevent the settlement of barnacles and tunicate larvae.[6]
Mechanisms of symbiosis
Various mechanisms are utilized in order to facilitate symbiotic relationships and to help these associates evolve alongside one another. By using horizontal gene transfer, certain genetic elements are able to pass from one organisms to another. In non-mating species, this helps with genetic differentiation and adaptive evolution.[7] An example of this is the sponge Astroclera willeyana which has a gene that is used in expressing spherulite-forming cells which has an origin in bacteria. Another example is the starlet sea anemone, Nematostella vectensis, which has genes from bacteria that have a role in producing UV radiation protection in the form of shikimic acid. Another way for symbiotic relationships to co-evolve is through genome erosion. This is a process where genes that are typically used during free-living periods aren't necessary because of the symbioses of the organisms. Without that gene, the organism is able to decrease the energy necessary for cell maintenance and replication.[7]
Types of symbiotic relationships
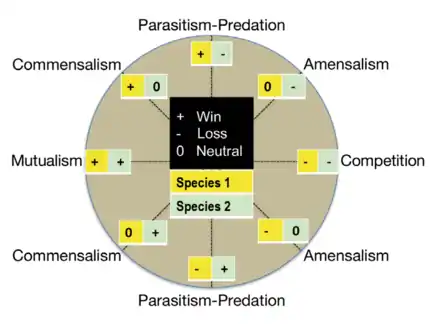
Among various types of symbiotic relationships, mutualism is where partners mutually benefit. Commensalism is a relationship where one partner receives a benefit while the other is not affected. There is parasitism, where one partner benefits while it is at the expense of the host.[9] And amensalism is a less common type of relationship where one organisms receives no benefit but the host still has negative ramifications. The relationships can be an ectosymbiont, a symbiont that survives by being attached to the surface of the host which includes areas such as the inner surfaces of the gut cavity or even the ducts of endocrine glands. Or it can be an endosymbiont which is a symbiont that lives within its host and can be known as an intracellular symbiont.[7] They are further classified by their dependence on their host and can be a facultative symbiont that can exist in a free living condition and is not dependent on its host. Or it can be an obligate symbiont which has adapted in such a way that it is not able to exit without the benefit it receives from its host. An example of an obligate symbioses is the relationship between microalgae and corals. The microalgae provides a large source of the coral diet[7]
Some symbiotic relationships
Coral reef symbiosis
The most notable display of marine symbiotic relationship would be coral. Coral reefs are home to a variety of dinoflagellate symbiont,[10] these symbionts give coral its bright coloring and are vital for the survival of the reef. The symbionts provide the coral with food in exchange for protection. If the waters warm or become too acidic, the symbionts are expelled, the coral bleaches and if conditions persist the coral will die. This in turn leads to the collapse of the entire reef ecosystem[10]
Bone eating worm symbiosis

Osedax, also called the bone eating worm is a siboglinid worm from polychaete genus. It was discovered in a whalefall community on the surface of bones, in the axis of Monterey Canyon, California, in 2002. Osedax lacks a mouth, a functional gut and a trophosome. But female osedax have a vascularized root system originating from their ovisac which contains heterotrophic endosymbiotic bacterial community dominated by γ-proteobacteria clade. They use the vascularized root system to access the whale bones. The endosymbionts help the host utilize nutrients from the whale bones.[11]
Hawaiian squid and Vibrio fischeri symbiosis
Hawaiian sepiolid squid Euprymna scolopes and bacterium Vibrio fischeri also show symbiosis. In this symbiosis, symbiont not only serve the host for defense, but also shapes the host morphology. Bioluminescent V. fischeri can be found in epithelial lined crypts of the light organ of the host. Symbiosis begins as soon as a newly hatched squid finds and houses V. fischeri bacteria.
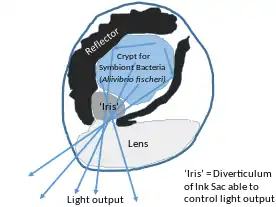
The symbiosis process begins when Peptidoglycan shed by the sea water bacteria comes in contact to the ciliated epithelial cells of the light organ. It induces mucus production in the cells. Mucus entraps bacterial cells. Antimicrobial peptides, nitric oxide and sialyted mucins in the mucus then selectively allow only V. fischeri which encode gene rscS to adhere and win over gram positive and other gram negative bacteria. The symbiotic bacteria are then guided up to the light organ via chemotaxis. After successful colonization, symbionts induce loss of mucus and ciliated sites to prevent further attachment of bacterial cells via MAMP (microbe associated molecular pattern) signalling. Also, they induce changes in protein expression in the host symbiotic tissues and modify both physiology and morphology of light organs. After bacterial cells divide and increase in population, they begin expressing enzyme luciferase as a result of quorum sensing. Luciferase enzymes produce bioluminescence.[12] Squids can then emit the luminescence from the light organ. Because Euprymna scolopes emerges only during night time, it helps them avoid predation. Bioluminescence allows them to camouflage with the light coming from moon and stars to ocean and avoid predators.[13]
Pompeii worm
Alvinella pompejana, the Pompeii worm is a polychaete, found in the far depths of the sea, typically found near hydrothermal vents. They were originally discovered by French researchers in the early 1980s.[14] They can grow as large as 5 inches long and are normally described as having pale gray coloring with red "tentacle-like" gills protruding from their heads. Their tails are most likely found in temperatures as high as 176 degrees Fahrenheit, while their heads, which stick out from the tubes they live in are only exposed to temperatures as high as 72 degrees Fahrenheit.[14] Its ability to survive the termpatures of hydrothermal vents lies in its symbiotic relationship with the bacteria that resides on its back. It forms a "fleece-like" protective covering. Mucous is secreted from glands on the back of the Pompeii worm in order to provide nutrients for the bacteria. Further study of the bacteria led to the discovery that they are chemolithotrophic.[14]
Hawaiian sea slug
Elysia rufescens grazes on Bryopsis sp., an alga that defends itself from predators by using peptide toxins with fatty acids, called kahalalides.[15] A bacterial obligate symbiont produces many defensive molecules, including kahalalides, in order to protect the alga. This bacteria is able to use substrates derived from the host in order to synthesize the toxins.[15] The Hawaiian Sea Slug grazes on the alga in order to accumulate kahalalide. This uptake of the toxin, which the slug is immune to, allows it to also become toxic to predators. This shared ability, both originating from the bacteria, provide protection within the marine ecosystems.
Marine sponges
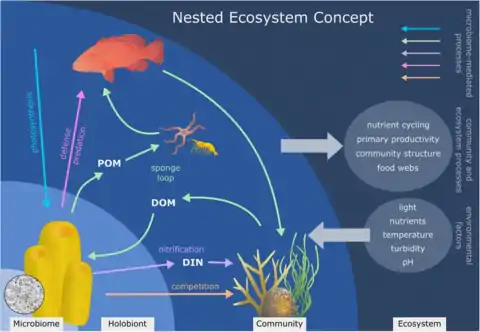
Besides a one to one symbiotic relationship, it is possible for a host to become symbiotic with a microbial consortia. In the case of the sponge (phylum Porifera), they are able to host a lot of wide range of microbial communities that can also be very specific. The microbial communities that form a symbiotic relationship with the sponge can actually comprise up to 35% of the biomass of its host.[17] The term for this specific symbiotic relationship, where a microbial consortia pairs with a host is called a holobiotic relationship. The sponge as well as the microbial community associated with it will produce a large range of secondary metabolites that help protect it against predators through mechanisms such as chemical defense.[18] Some of these relationships include endosymbionts within bacteriocyte cells, and cyanobacteria or microalgae found below the pinacoderm cell layer where they are able to receive the highest amount of light, used for phototrophy. They can host approximately 52 different microbial phyla and candidate phyla, including Alphaprotoebacteria, Actinobacteria, Chloroflexi, Nitrospirae, Cyanobacteria, the taxa Gamma-, and the candidate phylum Poribacteria, and Thaumarchaea.[18]
Endozoicomonas
This type of bacteria was first described in 2007.[19] It is able to form symbiotic relationships with a wide range of hosts in the marine environment such as cnidarians, poriferans, molluscs, annelids, tunicates, and fish. They are distributed through various marine zones from extreme depths to warm photic zones. Endozoicomonas is thought to acquisition nutrients from nitrogen/carbon recycling, methane/sulfur recycling, and synthesize amino acids and various other molecules necessary for life.[19] It was also found that it has a correlation to photosymbionts which provide carbon and sulfur to the bacteria from dimethylsulfopropionate (DMSP). They are also suspected to help regulate bacterial colonization of the host by using bioactive secondary metabolites or even probiotic mechanisms like limiting pathogenic bacteria by means of competitive exclusion. When Endozoicomonas is removed from the host, there are often signs of lesions on corals and disease.[19]
Chemosynthetic symbioses in ocean
Marine environment consists of a large number of chemosynthetic symbioses in different regions of the ocean: shallow-water coastal sediments, continental slope sediments, whale and wood falls, cold seeps and deep-sea hydrothermal vents. Organisms from seven phyla (ciliophora, porifera, platyhelminthes, nematoda, mollusca, annelida and arthropoda) are known to have chemosynthetic symbiosis till now. Some of them include nematode, tube worms, clam, sponge, hydrothermal vent shrimp, worms mollusc, mussels and so on. The symbionts can be ectosymbionts or endosymbionts. Some ectosymbionts are: symbionts of polychaete worm Alvinella which occur in their dorsal surface and symbionts occurring on the mouthparts and gill chamber of the vent shrimp Rimicaris. Endosymbionts include symbionts of gastropod snails which occur in their gill tissues. In the siboglinid tube worms of the groups Monilifera, Frenulata and Vestimentifera, symbionts can be found in an interior organ called trophosome.[20]
Most of the animals in deep-sea hydrothermal vents exist in a symbiotic relationship with chemosynthetic bacteria. These chemosynthetic bacteria are found to be methane or sulphur oxidizers. [21]
Microbial biotechnology
Marine invertebrates are the hosts of a wide spectrum of bioactive metabolites, which have vast potential as drugs and research tools.[22] In many cases, microbes aid in or are responsible for marine invertebrates natural products.[22] Certain marine microbes can provide insight into the biosynthesis mechanisms of natural products, which in turn could solve the current limitations on marine drug development.[5]
References
- Faust K and Raes J (2012) "Microbial interactions: From networks to models". Nat Rev Microbiol, 10: 538–550. doi:10.1038/nrmicro2832.
- Krabberød, A.K., Bjorbækmo, M.F., Shalchian-Tabrizi, K. and Logares, R. (2017) "Exploring the oceanic microeukaryotic interactome with metaomics approaches". Aquatic Microbial Ecology, 79(1): 1–12. doi:10.3354/ame01811.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Cavanaugh CM (February 1994). "Microbial Symbiosis: Patterns of Diversity in the Marine Environment". American Zoologist. 34 (1): 79–89. doi:10.1093/icb/34.1.79. JSTOR 3883820.
- Egan S, Gardiner M (2016). "Microbial Dysbiosis: Rethinking Disease in Marine Ecosystems". Frontiers in Microbiology. 7: 991. doi:10.3389/fmicb.2016.00991. PMC 4914501. PMID 27446031.
- Li Z (April 2009). "Advances in marine microbial symbionts in the china sea and related pharmaceutical metabolites". Marine Drugs. 7 (2): 113–29. doi:10.3390/md7020113. PMC 2707038. PMID 19597576.
- Armstrong E, Yan L, Boyd KG, Wright PC, Burgess JG (October 2001). "The symbiotic role of marine microbes on living surfaces". Hydrobiologia. 461 (1–3): 37–40. doi:10.1023/A:1012756913566. S2CID 33165122.
- Apprill A (January 2020). "The Role of Symbioses in the Adaptation and Stress Responses of Marine Organisms". Annual Review of Marine Science. 12 (1): 291–314. Bibcode:2020ARMS...12..291A. doi:10.1146/annurev-marine-010419-010641. PMID 31283425.
- Egan, S., Fukatsu, T. and Francino, M.P., 2020. Opportunities and Challenges to Microbial Symbiosis Research in the Microbiome Era. Frontiers in Microbiology, 11. doi:10.3389/fmicb.2020.01150.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Fukui S (May 2014). "Evolution of symbiosis with resource allocation from fecundity to survival". Die Naturwissenschaften. 101 (5): 437–46. Bibcode:2014NW....101..437F. doi:10.1007/s00114-014-1175-1. PMC 4012156. PMID 24744057.
- Baker A (November 2003). "Flexibility and Specificity in Coral-Algal Symbiosis: Diversity, Ecology, and Biogeography of Symbiodinium". Annual Review of Ecology, Evolution, and Systematics. 34: 661–689. doi:10.1146/annurev.ecolsys.34.011802.132417. JSTOR 30033790.
- Goffredi SK, Orphan VJ, Rouse GW, Jahnke L, Embaye T, Turk K, et al. (September 2005). "Evolutionary innovation: a bone-eating marine symbiosis". Environmental Microbiology. 7 (9): 1369–78. doi:10.1111/j.1462-2920.2005.00824.x. PMID 16104860.
- Schwartzman JA, Ruby EG (January 2016). "A conserved chemical dialog of mutualism: lessons from squid and vibrio". Microbes and Infection. 18 (1): 1–10. doi:10.1016/j.micinf.2015.08.016. PMC 4715918. PMID 26384815.
- Nyholm SV, McFall-Ngai MJ (August 2004). "The winnowing: establishing the squid-vibrio symbiosis". Nature Reviews. Microbiology. 2 (8): 632–42. doi:10.1038/nrmicro957. PMID 15263898. S2CID 21583331.
- "Pompeii Worm". Marine Symbiosis. Retrieved 2020-04-29.
- Zan J, Li Z, Tianero MD, Davis J, Hill RT, Donia MS (June 2019). "A microbial factory for defensive kahalalides in a tripartite marine symbiosis". Science. 364 (6445): eaaw6732. doi:10.1126/science.aaw6732. PMID 31196985. S2CID 189818260.
- Pita, L., Rix, L., Slaby, B.M., Franke, A. and Hentschel, U. (2018) "The sponge holobiont in a changing ocean: from microbes to ecosystems". Microbiome, 6(1): 46. doi:10.1186/s40168-018-0428-1.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Egan S, Thomas T (2015). "Editorial for: Microbial symbiosis of marine sessile hosts- diversity and function". Frontiers in Microbiology. 6: 585. doi:10.3389/fmicb.2015.00585. PMC 4468920. PMID 26136729.
- Webster NS, Thomas T (April 2016). "The Sponge Hologenome". mBio. 7 (2): e00135-16. doi:10.1128/mBio.00135-16. PMC 4850255. PMID 27103626.
- Neave MJ, Apprill A, Ferrier-Pagès C, Voolstra CR (October 2016). "Diversity and function of prevalent symbiotic marine bacteria in the genus Endozoicomonas". Applied Microbiology and Biotechnology. 100 (19): 8315–24. doi:10.1007/s00253-016-7777-0. PMC 5018254. PMID 27557714.
- Dubilier N, Bergin C, Lott C (October 2008). "Symbiotic diversity in marine animals: the art of harnessing chemosynthesis". Nature Reviews. Microbiology. 6 (10): 725–40. doi:10.1038/nrmicro1992. PMID 18794911. S2CID 3622420.
- Petersen JM, Zielinski FU, Pape T, Seifert R, Moraru C, Amann R, et al. (August 2011). "Hydrogen is an energy source for hydrothermal vent symbioses". Nature. 476 (7359): 176–80. Bibcode:2011Natur.476..176P. doi:10.1038/nature10325. PMID 21833083. S2CID 25578.
- Haygood MG, Schmidt EW, Davidson SK, Faulkner DJ (August 1999). "Microbial symbionts of marine invertebrates: opportunities for microbial biotechnology" (PDF). Journal of Molecular Microbiology and Biotechnology. 1 (1): 33–43. PMID 10941782.