Piprozolin
Piprozolin (or piprozoline) is a medication for bile therapy.[1][2]
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| ECHA InfoCard | 100.037.512 |
| Chemical and physical data | |
| Formula | C14H22N2O3S |
| Molar mass | 298.40 g·mol−1 |
InChI
| |
| (verify) | |
Synthesis
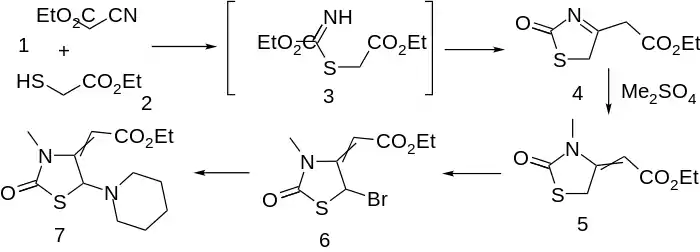
Compared to fenclozic acid, piprozolin shows choleretic rather than anti-inflammatory activity. That is, the compound is useful in those conditions where the flow of bile is to be increased.
Condensation of ethyl mercaptoacetate with ethyl cyanoacetate leads to thiazolinone (4); an intermediate such as 3, involving addition of the mercaptide to the nitrile function can reasonably be invoked. M/ethylation with di(m)ethyl sulfate proceeds on nitrogen with the concomitant shift of the enamine to afford olefin (5). Bromination of the active methylene (6) followed by displacement of halogen by piperidine affords the choleretic piprozolin (7).
References
- Koss FW, Vollmer KO, Herrmann M (August 1972). "[Research with experimental animals on the mechanism of action of the new choleretic, 3-ethyl-4-oxo-5-piperidino-delta 2, alpha-thiazolidine-acetic acid ethyl ester (Piprozolin)]". Archives Internationales de Pharmacodynamie et de Therapie. 198 (2): 333–46. PMID 5074733.
- "Piprozolin". Drugs-about.com.
- Satzinger (1975). "Substituierte 2-Methylen-thiazolidone-(4)". Justus Liebigs Annalen der Chemie. 665: 150–165. doi:10.1002/jlac.19636650118.
- DE 2414345, Satzinger G, Herrmann M, Vollmer K, issued 1975, assigned to Goeecke
- US 3971794, Satzinger G, Herrmann M, Vollmer K, issued 1976, assigned to Warner-Lambert
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.