Toluene
Toluene (/ˈtɒl.juiːn/), also known as toluol (/ˈtɒl.ju.ɒl, -ɔːl, -oʊl/),[7][8] is a substituted aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) attached to a phenyl group. As such, its systematic IUPAC name is methylbenzene. Toluene is predominantly used as an industrial feedstock and a solvent.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Toluene[1] | |||
| Systematic IUPAC name
Methylbenzene | |||
| Other names
Phenyl methane Toluol Anisen | |||
| Identifiers | |||
3D model (JSmol) |
|||
| Abbreviations | PhMe MePh BnH Tol | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.003.297 | ||
IUPHAR/BPS |
|||
| KEGG | |||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C7H8 | |||
| Molar mass | 92.141 g·mol−1 | ||
| Appearance | Colorless liquid[2] | ||
| Odor | sweet, pungent, benzene-like[3] | ||
| Density | 0.87 g/mL (20 °C)[2] | ||
| Melting point | −95 °C (−139 °F; 178 K)[2] | ||
| Boiling point | 111 °C (232 °F; 384 K)[2] | ||
| 0.52 g/L (20 °C)[2] | |||
| log P | 2.68[4] | ||
| Vapor pressure | 2.8 kPa (20 °C)[3] | ||
| −66.11·10−6 cm3/mol | |||
Refractive index (nD) |
1.497 (20 °C) | ||
| Viscosity | 0.590 cP (20 °C) | ||
| Structure | |||
Dipole moment |
0.36 D | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards |
highly flammable | ||
| GHS labelling: | |||
   | |||
| Danger | |||
Hazard statements |
H225, H304, H315, H336, H361d, H373 | ||
Precautionary statements |
P210, P240, P301+P310, P302+P352, P308+P313, P314, P403+P233 | ||
| NFPA 704 (fire diamond) | |||
| Flash point | 4 °C (39 °F; 277 K)[2] | ||
| Explosive limits | 1.1–7.1%[3] | ||
Threshold limit value (TLV) |
50 mL/m3, 190 mg/m3 | ||
| Lethal dose or concentration (LD, LC): | |||
LC50 (median concentration) |
>26700 ppm (rat, 1 h) 400 ppm (mouse, 24 h)[5] | ||
LCLo (lowest published) |
55,000 ppm (rabbit, 40 min)[5] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
TWA 200 ppm C 300 ppm 500 ppm (10-minute maximum peak)[3] | ||
REL (Recommended) |
TWA 100 ppm (375 mg/m3) ST 150 ppm (560 mg/m3)[3] | ||
IDLH (Immediate danger) |
500 ppm[3] | ||
| Safety data sheet (SDS) | SIRI.org | ||
| Related compounds | |||
Related aromatic hydrocarbons |
benzene xylene naphthalene | ||
Related compounds |
methylcyclohexane | ||
| Supplementary data page | |||
| Toluene (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
As the solvent in some types of paint thinner, permanent markers, contact cement and certain types of glue, toluene is sometimes used as a recreational inhalant[9] and has the potential of causing severe neurological harm.[10][11]
History
The compound was first isolated in 1837 through a distillation of pine oil by the Polish chemist Filip Walter, who named it rétinnaphte.[12] In 1841, French chemist Henri Étienne Sainte-Claire Deville isolated a hydrocarbon from balsam of Tolu (an aromatic extract from the tropical Colombian tree Myroxylon balsamum), which Deville recognized as similar to Walter's rétinnaphte and to benzene; hence he called the new hydrocarbon benzoène.[13] In 1843, Jöns Jacob Berzelius recommended the name toluin.[14] In 1850, French chemist Auguste Cahours isolated from a distillate of wood a hydrocarbon which he recognized as similar to Deville's benzoène and which Cahours named toluène.[15]
Chemical properties
Toluene reacts as a normal aromatic hydrocarbon in electrophilic aromatic substitution.[16][17][18] Because the methyl group has greater electron-releasing properties than a hydrogen atom in the same position, toluene is more reactive than benzene toward electrophiles. It undergoes sulfonation to give p-toluenesulfonic acid, and chlorination by Cl2 in the presence of FeCl3 to give ortho and para isomers of chlorotoluene.
Importantly, the methyl side chain in toluene is susceptible to oxidation. Toluene reacts with potassium permanganate to yield benzoic acid, and with chromyl chloride to yield benzaldehyde (Étard reaction).
The C-H bonds of the methyl group in toluene are benzylic, which means that they are weaker than C-H bonds in simpler alkanes. Reflecting this weakness, the methyl group in toluene undergoes halogenation under free radical conditions. For example, when heated with N-bromosuccinimide (NBS) in the presence of AIBN, toluene converts to benzyl bromide. The same conversion can be effected with elemental bromine in the presence of UV light or even sunlight.
Toluene may also be brominated by treating it with HBr and H2O2 in the presence of light.[19]
- C6H5CH3 + Br2 → C6H5CH2Br + HBr
- C6H5CH2Br + Br2 → C6H5CHBr2 + HBr
The methyl group in toluene undergoes deprotonation only with very strong bases; its pKa is estimated to be approximately 41.[20] Complete hydrogenation of toluene gives methylcyclohexane. The reaction requires a high pressure of hydrogen and a catalyst.
Miscibility
Toluene is miscible (soluble in all proportions) with ethanol, benzene, diethyl ether, acetone, chloroform, glacial acetic acid and carbon disulfide, but immiscible with water.[21]
Production
Toluene occurs naturally at low levels in crude oil and is a byproduct in the production of gasoline by a catalytic reformer or ethylene cracker. It is also a byproduct of the production of coke from coal. Final separation and purification is done by any of the distillation or solvent extraction processes used for BTX aromatics (benzene, toluene, and xylene isomers).[22]
Uses
Precursor to benzene and xylene
Toluene is mainly used as a precursor to benzene via hydrodealkylation:
- C6H5CH3 + H2 → C6H6 + CH4
The second ranked application involves its disproportionation to a mixture of benzene and xylene.[22]
Nitration
Nitration of toluene gives mono-, di-, and trinitrotoluene, all of which are widely used. Dinitrotoluene is the precursor to toluene diisocyanate, which used in the manufacture of polyurethane foam. Trinitrotoluene is the explosive typically abbreviated TNT.
Oxidation
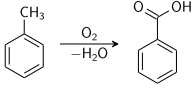
Benzoic acid and benzaldehyde are produced commercially by partial oxidation of toluene with oxygen. Typical catalysts include cobalt or manganese naphthenates.[23]
Solvent
Toluene is a common solvent, e.g. for paints, paint thinners, silicone sealants,[24] many chemical reactants, rubber, printing ink, adhesives (glues), lacquers, leather tanners, and disinfectants.[22]
Fuel
Toluene can be used as an octane booster in gasoline fuels for internal combustion engines as well as jet fuel. Toluene at 86% by volume fuelled all the turbocharged engines in Formula One during the 1980s, first pioneered by the Honda team. The remaining 14% was a "filler" of n-heptane, to reduce the octane rating to meet Formula One fuel restrictions. Toluene at 100% can be used as a fuel for both two-stroke and four-stroke engines; however, due to the density of the fuel and other factors, the fuel does not vaporize easily unless preheated to 70 °C (158 °F). Honda solved this problem in their Formula One cars by routing the fuel lines through a heat exchanger, drawing energy from the water in the cooling system to heat the fuel.[25]
In Australia in 2003, toluene was found to have been illegally combined with petrol in fuel outlets for sale as standard vehicular fuel. Toluene incurs no fuel excise tax, while other fuels are taxed at more than 40%, providing a greater profit margin for fuel suppliers. The extent of toluene substitution has not been determined.[26][27]
Niche applications
In the laboratory, toluene is used as a solvent for carbon nanomaterials, including nanotubes and fullerenes, and it can also be used as a fullerene indicator. The color of the toluene solution of C60 is bright purple. Toluene is used as a cement for fine polystyrene kits (by dissolving and then fusing surfaces) as it can be applied very precisely by brush and contains none of the bulk of an adhesive. Toluene can be used to break open red blood cells in order to extract hemoglobin in biochemistry experiments. Toluene has also been used as a coolant for its good heat transfer capabilities in sodium cold traps used in nuclear reactor system loops. Toluene had also been used in the process of removing the cocaine from coca leaves in the production of Coca-Cola syrup.[28]
Toxicology and metabolism
The environmental and toxicological effects of toluene have been extensively studied.[29] Inhalation of toluene in low to moderate levels can cause tiredness, confusion, weakness, drunken-type actions, memory loss, nausea, loss of appetite, hearing loss,[30][31] and colour vision loss.[32] Some of these symptoms usually disappear when exposure is stopped. Inhaling high levels of toluene in a short time may cause light-headedness, nausea, or sleepiness, unconsciousness, and even death.[33][34] Toluene is, however, much less toxic than benzene, and as a consequence, largely replaced it as an aromatic solvent in chemical preparation. The US Environmental Protection Agency (EPA) states that the carcinogenic potential of toluene cannot be evaluated due to insufficient information.[35] In 2013, worldwide sales of toluene amounted to about 24.5 billion US-dollars.[36]
Similar to many other solvents such as 1,1,1-trichloroethane and some alkylbenzenes, toluene has been shown to act as a non-competitive NMDA receptor antagonist and GABAA receptor positive allosteric modulator.[37] Additionally, toluene has been shown to display antidepressant-like effects in rodents in the forced swim test (FST) and the tail suspension test (TST),[37] likely due to its NMDA antagonist properties.
Toluene is sometimes used as a recreational inhalant ("glue sniffing"), likely on account of its euphoric and dissociative effects.[37]
Toluene inhibits excitatory ion channels such as the NMDA receptor, nicotinic acetylcholine receptor, and the serotonin 5-HT3 receptor. It also potentiates the function of inhibitory ion channels, such as the GABAA and glycine receptors. In addition, toluene disrupts voltage-gated calcium channels and ATP-gated ion channels.[38]
Recreational use
Toluene is used as an intoxicative inhalant in a manner unintended by manufacturers. People inhale toluene-containing products (e.g., paint thinner, contact cement, model glue, etc.) for its intoxicating effect. The possession and use of toluene and products containing it are regulated in many jurisdictions, for the supposed reason of preventing minors from obtaining these products for recreational drug purposes. As of 2007, 24 U.S. states had laws penalizing use, possession with intent to use, and/or distribution of such inhalants.[39] In 2005 the European Union banned the general sale of products consisting of greater than 0.5% toluene.[40]
References
- Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 139. doi:10.1039/9781849733069-00130. ISBN 978-0-85404-182-4.
Toluene and xylene are preferred IUPAC names, but are not freely substitutable; toluene is substitutable under certain conditions, but only for general nomenclature (see P-15.1.8 for a general substitution rules for retained names).
- Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- NIOSH Pocket Guide to Chemical Hazards. "#0619". National Institute for Occupational Safety and Health (NIOSH).
- "toluene_msds". Archived from the original on 2022-09-13. Retrieved 2018-04-11.
- "Toluene". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- "NFPA Chemicals". New Environment, Inc. Archived from the original on 2021-11-14. Retrieved 2015-03-13.
- "toluol". Dictionary.com Unabridged (Online). n.d.
- "toluene". The American Heritage Dictionary of the English Language (5th ed.). HarperCollins.
- McKeown NJ (Feb 1, 2015). Tarabar A (ed.). "Toluene Toxicity, Background, Pathophysiology, Epidemiology". WebMD Health Professional Network. Archived from the original on March 9, 2016. Retrieved March 22, 2016.
{{cite journal}}: Cite journal requires|journal=(help) - Streicher HZ, Gabow PA, Moss AH, Kono D, Kaehny WD (June 1981). "Syndromes of toluene sniffing in adults". Annals of Internal Medicine. 94 (6): 758–62. doi:10.7326/0003-4819-94-6-758. PMID 7235417.
- Devathasan G, Low D, Teoh PC, Wan SH, Wong PK (February 1984). "Complications of chronic glue (toluene) abuse in adolescents". Australian and New Zealand Journal of Medicine. 14 (1): 39–43. doi:10.1111/j.1445-5994.1984.tb03583.x. PMID 6087782.
- See:
- Pelletier and Walter (1837) "Examen des produits provenant du traitement de la résine dans la fabrication du gaz pour l'éclairage" Archived 2016-10-21 at the Wayback Machine (Examination of products arising from the treatment of resin during the production of illuminating gas), Comptes rendus, 4 : 898–899.
- Pelletier and Philippe Walter (1838) "Examen des produits provenant du traitement de la résine dans la fabrication du gaz pour l'éclairage," Archived 2022-09-13 at the Wayback Machine Annales de Chimie et de Physique, 2nd series, 67 : 269-303. Toluene is named on pp. 278-279: "Nous désignerons la substance qui nous occupe par le nom de rétinnaphte, qui rappelle son origine et ses propriétés physiques (ρητίνη-νάφτα)." (We will designate the substance that occupies us by the name of rétinnaphte, which recalls its origin and its physical properties (ρητίνη-νάφτα [resin-naphtha]).
- See:
- Deville (1841) "Recherches sur les résines. Étude du baume de Tolu" Archived 2016-10-21 at the Wayback Machine (Investigations of resins. Study of Tolu balsam), Comptes rendus, 13 : 476–478.
- H. Deville (1841) "Recherches chimiques sur les résines; Premier mémoire" Archived 2021-05-02 at the Wayback Machine (Chemical investigations of resins; first memoir), Annales de Chimie et de Physique, 3rd series, 3 : 151-195. Deville names toluene on p. 170: "J'ai adopté, pour le corps qui m'occupe dans ce moment, le nom de benzoène, qui rappelle, dans les baumes dont il provient, ce caractère presque générique qui est de contenir de l'acide benzoïque." (I've adopted, for this substance that occupies me at the moment, the name benzoène, which recalls, in the balsams from which it comes, that character which is contained in benzoic acid.)
- Wisniak J (2004). "Henri Étienne Sainte-Claire Deville: A physician turned metallurgist". Journal of Materials Engineering and Performance. 13 (2): 117–118. Bibcode:2004JMEP...13..117W. doi:10.1361/10599490418271. S2CID 95058552.
- Jacob Berzelius (1843) Jahres Berichte, 22 : 353-354. Archived 2022-09-13 at the Wayback Machine.
- See:
- Cahours A (1850). "Recherches sur les huiles légères obtenues dans la distillation du bois" [Investigations of light oils obtained by the distillation of wood]. Comptes Rendus (in French). 30: 319–323 (320). Archived from the original on 2016-03-01. Retrieved 2015-08-02.
- Wisniak J (October 2013). "Auguste André Thomas Cahours". Educación Química. 24 (4): 451–460. doi:10.1016/S0187-893X(13)72500-X.
- Vogel AS, Furniss BS, Hannaford AJ, Tatchell AR, Smith PW (1989). Vogel's Textbook of Practical Organic Chemistry (5th ed.). New York: Longman/Wiley.
- Wade LG (2003). Organic Chemistry (5th ed.). Upper Saddle River, New Jersey: Prentice Hall. p. 871.
- March J (1992). Advanced Organic Chemistry (4th ed.). New York: Wiley. p. 723.
- Podgoršek A, Stavber S, Zupan M, Iskra J (2006). "Free Radical Bromination by the H2O2–HBr System on water". Tetrahedron Letters. 47 (40): 7245–7247. doi:10.1016/j.tetlet.2006.07.109.
- Henry Hsieh, Roderic P. Quirk. Anionic Polymerization: Principles and Practical Applications. p. 41.
- "Toluene, Semiconductor Grade, 99% min, Thermo Scientific | Fisher Scientific". www.fishersci.com. Retrieved 2022-04-26.
- Jörg F, Ulrich G, Simo TA (2005). "Toluene". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a27_147.pub2.
- Wade LG (2014). Organic Chemistry (Pearson new international ed.). Harlow: Pearson Education Limited. p. 985. ISBN 978-1-292-02165-2.
- "Dual cure, low-solvent silicone pressure sensitive adhesives - General Electric Company". Archived from the original on 2012-10-04. Retrieved 2008-02-15.
- Honda Formula One Turbo-charged V-6 1.5L Engine (PDF). SAE International Congress and Exposition. February 27 – March 3, 1989. Archived (PDF) from the original on September 11, 2017. Retrieved September 11, 2017.
- "Scam on petrol sparks spot tests". Liberty Oil. Archived from the original on 3 March 2016.
- "The World Today Archive - Authorities yet to acknowledge petrol scam problem". Australian Broadcasting Corporation. Archived from the original on 2012-11-10. Retrieved 2009-09-04.
- Merory J (1968). Food Flavorings: Composition, Manufacture and Use (2nd ed.). Westport, CT: AVI Publishing Company, Inc..
- Hogan CM (2011), "Sulfur", in Jorgensen A, Cleveland CJ (eds.), Encyclopedia of Earth, Washington DC: National Council for Science and the Environment, archived from the original on 28 October 2012, retrieved 26 October 2012,
Sulfur is insoluble in water, but soluble in carbon disulfide, somewhat soluble in other non-polar organic solvents such as the aromatics benzene and toluene.
- Chang SJ, Chen CJ, Lien CH, Sung FC (August 2006). "Hearing loss in workers exposed to toluene and noise". Environmental Health Perspectives. 114 (8): 1283–6. doi:10.1289/ehp.8959. PMC 1552019. PMID 16882540.
- Morata TC, Nylén P, Johnson AC, Dunn DE (1995). "Auditory and vestibular functions after single or combined exposure to toluene: a review". Archives of Toxicology. 69 (7): 431–43. doi:10.1007/s002040050196. PMID 8526738. S2CID 22919141.
- Kishi R, Eguchi T, Yuasa J, Katakura Y, Arata Y, Harabuchi I, et al. (January 2001). "Effects of low-level occupational exposure to styrene on color vision: dose relation with a urinary metabolite". Environmental Research. 85 (1): 25–30. Bibcode:2001ER.....85...25K. doi:10.1006/enrs.2000.4227. PMID 11161648.
- "Health Effects of Toluene" Archived 2010-11-25 at the Wayback Machine, Canadian Centre for Occupational Health and Safety.
- "Toluene Toxicity Physiologic Effects" Archived 2016-10-12 at the Wayback Machine, Agency for Toxic Substances and Disease Registry.
- Archived 2015-03-06 at the Wayback Machine, EPA
- Ceresana. "Toluene – Study: Market, Analysis, Trends - Ceresana". Archived from the original on 2017-04-29. Retrieved 2015-04-14.
- Cruz SL, Soberanes-Chávez P, Páez-Martinez N, López-Rubalcava C (June 2009). "Toluene has antidepressant-like actions in two animal models used for the screening of antidepressant drugs". Psychopharmacology. 204 (2): 279–86. doi:10.1007/s00213-009-1462-2. PMID 19151967. S2CID 2235023.
- "Toluene". Archived from the original on 2019-02-16. Retrieved 2019-02-15.
- Spigel S (8 July 2009). "State Laws on Inhalant Use". Archived from the original on 25 February 2015. Retrieved 13 April 2015.
- "EU sets 0.1% limit on use of toluene, TCB". ICIS. Reed Business Information. 24 September 2005. Archived from the original on 18 July 2018. Retrieved 18 July 2018.
- Prenafeta-Boldu FX, Kuhn A, Luykx DM, Anke H, van Groenestijn JW, de Bont JA (April 2001). "Isolation and characterisation of fungi growing on volatile aromatic hydrocarbons as their sole carbon and energy source". Mycological Research. 105 (4): 477–484. doi:10.1017/S0953756201003719. Archived from the original on 2017-09-22. Retrieved 2018-04-20.
External links
- ATSDR – Case Studies in Environmental Medicine: Toluene Toxicity U.S. Department of Health and Human Services (public domain)
- American Industrial Hygiene Association, The Ear Poisons, The Synergist, November 2018.
- Toluene CDC – NIOSH Workplace Safety and Health Topic (DHHS)
- OSHA-NIOSH 2018. Preventing Hearing Loss Caused by Chemical (Ototoxicity) and Noise Exposure Safety and Health Information Bulletin (SHIB), Occupational Safety and Health Administration and the National Institute for Occupational Safety and Health. SHIB 03-08-2018. DHHS (NIOSH) Publication No. 2018-124.