Alkanes, also called paraffins, are a class of hydrocarbons that are fully saturated with hydrogen. They contain no double or triple bonds in their carbon skeletons and, therefore, have the maximum number of carbon to hydrogen covalent bonds. This is in contrast to alkenes and alkynes, which contain double and triple bonds and are known as unsaturated hydrocarbons.
Structure of Alkanes
Alkanes have the general formula CnH2n+2. For example, an alkane with 2 (n) carbon atoms, will have 6 (2n + 2) hydrogen atoms. Their adjacent atoms are connected with sigma bonds and form tetrahedral centers around the carbon atoms. As these bonds are all single bonds, there is free rotation around all connections. Each carbon atom has four bonds (either C-H or C-C bonds), and each hydrogen atom is joined to a carbon atom (H-C bonds). A series of linked carbon atoms is known as the carbon skeleton or carbon backbone. The number of carbon atoms is used to define the size of the alkane (e.g., C2-alkane).
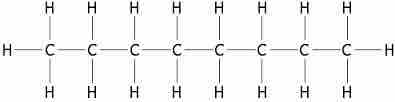
The octane molecule
The number of carbon atoms (n) in the octane molecule is 8. The number of hydrogen atoms (2n +2) is 18.
An alkyl group, generally abbreviated with the symbol R, is a functional group or side-chain that, like an alkane, consists solely of single-bonded carbon and hydrogen atoms; for example, R could represent a methyl or ethyl group. An alkyl group is a piece of a molecule with the general formula (CH3)n, where n is any integer. For example, a methyl group (CH3) is a fragment of a methane molecule (CH4). In this example, n=1.
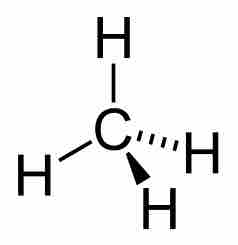
Methane
The chemical structure of methane, a simple alkane.
The simplest possible alkane is methane (CH4). Saturated oils and waxes are examples of larger alkanes where the number of carbons in the carbon backbone is greater than ten.
In linear alkanes, the carbon atoms are joined in a snake-like structure. In branched alkanes, the carbon backbone splits off in one or more directions. In cyclic alkanes, the carbon backbone is linked so as to form a loop. Cyclic and branched alkanes will be discussed in greater detail in subsequent sections.
Nomenclature for Alkanes
Alkanes are named with the suffix "-ane" following the hydrocarbon prefixes. The series contains methane (CH4), ethane (C2H6), propane (C3H8), butane (C4H10), pentane (C5H12), and so on. For carbon chains with length of 6, 7, 8, 9, and 10 atoms, the prefixes are "hex-," "hept-," "oct-," "non-," and "dec-," respectively.
For the higher molecular weight compounds, the four bonds formed by carbon allow for a number of variations on the carbon skeleton. These multiple forms, which share the same molecular formula, are known as isomers. The prefix "n-," for normal, is reserved for the linear, unbranched forms of these alkanes .
Properties of Alkanes
The smaller members of the alkane family are gases, while the larger compounds are liquid and solid compounds. They are commonly found in fuel sources, like natural gas and petroleum. The solid compounds are typically waxy in texture.
Alkanes have a number of industrial applications beyond fuels, including uses in cosmetics and plastics. Alkanes are generally less reactive than alkenes and alkynes because they lack the more reactive double and triple bonds. However, they do participate in reactions with oxygen (combustion) and halogens.