Central Cancer Registry Domain Diagram
A domain diagram is a skeleton class diagram that consists of—
- A group of classes and interfaces that reflect important entities of the business domain of the system being modeled.
- The relationships between the classes and interfaces.
The NPCR–AERRO Central Cancer Registry Domain Diagram shows the interactions between the entities involved in central cancer registry functions. It shows the formation of a cancer abstract from the time individual event reports are generated at different data sources to the time the cancer abstract is stored in the central cancer registry and made available to institutions and national programs for research.
A text description of the diagram and legend may be found below. For information about reading diagrams, see Diagram Conventions.
- Central Cancer Registry
A central cancer registry (CCR) collects, processes, and analyzes data on all cancer cases diagnosed. Each state in the United States has a CCR. This organization refers to other CCRs and is a business actor external to the CCR business process. It interacts with the CCR when the CCR performs interstate data exchange and provides data for use by others. - Cancer Information
Facts or data that describe the occurrence, diagnosis, and treatment of cancer. - NPCR/SEER/NAACCR
The Centers for Disease Control and Prevention’s National Program of Cancer Registries (NPCR), the Surveillance, Epidemiology and End Results (SEER) Program of the National Cancer Institute, and the North American Association of Central Cancer Registries (NAACCR). - Research
A detailed study of data relating to the occurrence, diagnosis, and treatment of cancer. - Central Registry Registrar
A data management professional who validates event reports; links patients and tumors; performs consolidation, audits, quality assurance, and rapid case ascertainment; links to external data sources to improve data and for research; conducts death clearance, follow-up, and interstate data exchange; responds to calls for data; and provides data for use by others. - Cancer Case (Consolidated Record)
A summary of all event reports submitted by reporting facilities. It contains the final best information regarding a patient and his or her cancer and includes patient demographic, medical, staging, treatment, and service information. - Event Report
An electronic submission of data, usually the electronic health record or a cancer registry. - Non-Registry Event Report
An electronic submission of data from a data source other than a cancer registry. - Registry Event Report (Abstract)
An abstract is an abbreviated record that identifies the patient, disease, treatment, and disease process from the time of diagnosis until the patient’s death. It usually is divided into sections: patient identification, cancer identification, stage of disease at diagnosis, first course of treatment, recurrence, and follow-up. The abstract is the basis for the rest of the registry’s functions; without this summary of a patient’s cancer experience, no other registry function can take place. - Routine Event Report
A report submitted by a data source relating to a patient with cancer, such as a pathology report, discharge summary, insurance claim, or death certificate. - Corrected Event Report
An event report that corrects an error in a previously submitted event report. - Updated Event Report
An event report that provides additional information on a previously submitted event report. - Rapid Event Report
An event report transmitted in real time to notify a central cancer registry of a cancer diagnosis quickly, usually to include a patient in a research study. - Reporting Facility
Provides data to the cancer registry. It is responsible for the accuracy of the data and for providing corrections and updates. The two types of reporting facilities in the central cancer registry domain are healthcare facilities and non-healthcare facilities. - Heathcare (Hospital) Facility Data Source
An organization that provides medical services to patients, and contributes clinical as well as other information to a cancer registry. - Non-Healthcare (Non-Hospital) Facility Data Source
An organization that provides non-medical information to a cancer registry. - Hospital
A healthcare facility that provides inpatient and outpatient diagnostic, treatment, and palliative care services. It may serve a community or regional population or serve as a teaching and referral center. A hospital may seek approval for its cancer program from the American College of Surgeons Commission on Cancer, whose program standards require operation of a hospital cancer registry. - Independent Healthcare Practitioners
Solo or group physician practices, usually focused on a specialty area of medical practice such as internal medicine, general surgery, or urology. Their medical records identify cancer patients and provide cancer-specific treatment data and patient follow-up information. Physician practices may be required to report cancer cases to the central registry, to respond to inquiries from the central registry, or to allow central registry access to their medical records. - Radiation Oncology Center
The department that provides curative, adjuvant, or palliative cancer treatment using radiation to control malignant cells. Radiation may be given as external beam radiotherapy, brachytherapy or implantation of radioactive sources, or injection or ingestion of radioactive materials. Radiation oncology documentation, which may be maintained separate from the hospital medical record, includes pre-treatment consultation summarizing the cancer diagnosis and treatment to date, treatment planning and daily dose delivery, treatment summary, and patient follow-up visits. - Freestanding Surgery Center
A surgical facility that is independent from an acute care facility. - Freestanding Path Labs
Pathology laboratories that operate independently from other healthcare facilities, whose clients may include physician and clinic practices as well as hospitals. They examine organs, tissues, cells, and bodily fluids removed from patients for the investigation and diagnosis of disease, and conduct autopsies to study disease processes and to determine cause of death. Pathology reports include the type of material examined, body location from which the specimen was taken, gross and microscopic description and evaluation of tissues, components of bodily fluids, and diagnosis. Pathology laboratories may be required to report cancer cases to the central registry, to respond to inquiries from the central registry, or to allow central registry access to pathology records. - Nursing Home
A long-term care and end-of-life care facility. Cancer patients may be diagnosed while permanently or temporarily resident in a nursing home. It may be required to respond to inquiries from the central registry, or to allow central registry access to its medical records, particularly for case ascertainment on patients who died with a cancer diagnosis identified only through death certification information. - IHS/Local Tribe Clinic
The Indian Health Service (IHS) provides comprehensive health services through IHS and tribally contracted hospitals, health centers, school health centers, and health stations. The health services provided include medical, dental, and environmental health programs. Central registries are required to link data with the IHS database to improve the identification and surveillance of American Indians and Alaska Natives diagnosed with cancer. - Prison
Incarcerated patients’ medical records may document routine care delivered to a prison population or individual care received at a prison medical center. Cancer diagnostic and treatment information for prisoners generally is available through the hospital that provides non-routine medical services to the prison. Follow-up information may be obtained from the healthcare provider. - Office of Medical Examiner
A public official who investigates any death not due to natural causes. - Hospital Registry
A hospital cancer registry collects information on all cancer patients who use the services of a hospital. It may be required to report cancer cases to the central registry, to respond to inquiries from the central registry, or to allow central registry access to its records. - Oncology Clinic
An ambulatory care unit responsible for staging, medical treatment, and follow-up of cancer patients in a hospital. A clinic may focus on a particular cancer site such as breast cancer, provide a centralized setting for chemotherapy administration, or coordinate all services provided to oncology patients throughout the facility. Medical oncology documentation varies according to the services provided and may be separate from the hospital medical record. - Hospital Pathology
The hospital department that examines organs, tissues, cells, and bodily fluids removed from patients for the investigation and diagnosis of disease, and conducts autopsies to study disease processes and to determine cause of death. Pathology reports include the type of material examined, body location from which the specimen was taken, gross and microscopic description and evaluation of tissues, components of bodily fluids, and diagnosis. A pathology laboratory may be required to report cancer cases to the central registry, to respond to inquiries from the central registry, or to allow central registry access to pathology records. - Claims (Medicare, Medicaid, Private, Billing)
The department responsible for billing and collecting payment for health care services rendered by the facility. The business office provides the information on the financial statement submitted for payment. - Hospital Disease Index
A numerically sequenced list of diseases and conditions diagnosed in hospital patients. Diseases and conditions identified in patient medical records are coded using a standard classification system such as ICD-9-CM or CPT (Current Procedural Terminology). The disease index, compiled from these codes, is a casefinding source for the cancer registry. - e-Prescriptions
e-Prescribing is the process of prescribing medication using a computerized physician order entry system linked with clinical decision support that can send prescriptions or prescription-related information to pharmacies electronically. - Diagnostic Imaging
Independent healthcare facilities whose clients may include physician and clinic practices as well as hospitals. They create images of body organs and structures for the purposes of diagnosing disease, evaluating disease progression, and monitoring the effects of treatment. Imaging information generally is provided to central cancer registries through reporting procedures established with physician offices and treatment facilities. - Health Insurance Plan
Medical practice managed by an insurance plan, in which subscribers generally receive primary care at clinics owned by the plan, with referral to outside specialists as needed. A plan maintains consolidated medical records for its patients who receive medical care from multiple facilities and may provide cancer information on shared patients to hospital registries or establish its own registry database for monitoring patient care and outcomes. A plan may be required to report cancer cases to the central registry, to respond to inquiries from the central registry, or to allow central registry access to its medical records. - State Registry
A population-based registry that identifies cancer cases not reported by outside entities, abstracts missing cancer information, and consolidates cancer information from multiple sources into a single record. Registry staff monitor the completeness of reporting, maintain relationships with reporting entities, know coding systems and rules, and help reporting entities refine casefinding and abstracting practices. - State Health Department
A state government agency that promotes the health of state residents. It usually applies for and manages federal grants to support federally mandated health-related programs such as the National Breast and Cervical Cancer Early Detection Program (NBCCEDP). Central registries are required to link their data with the NBCCEDP database to identify missing cases and improve data quality in both databases. - Bureau of Vital Statistics
An agency within a state government that collects, maintains, and distributes information on births and deaths for state residents. It provides demographic and follow-up data for patients in the central registry database, including race, ethnicity, occupation, and date and cause of death. - National Death Index
A central computerized index of death record information aggregated from state vital statistics offices, beginning with 1979 deaths. Established as a resource to aid epidemiologists and other health and medical investigators with mortality ascertainment activities, the index provides information on the date, location, and cause of death. - Census Tract Database
A database maintained by the United States Census Bureau containing demographic and economic data by census tract, county, and state divisions. It provides information for generating a measure of socioeconomic status for cancer patients by address at diagnosis. - Voter Registration
Rolls of registered voters within a state, maintained by local jurisdictions, containing the voter’s name, date of registration, and address at registration. Voter registration provides information for determination and confirmation of address at diagnosis. - Department of Motor Vehicles (DMV)
A state agency that issues driver licenses and maintains traffic offense data. It provides information for the determination or confirmation of date of birth and address at diagnosis. - Health maintenance organization (HMO)
A type of health insurance plan. - Center for Medicare and Medicaid Services (CMS)
A type of health insurance plan. - State/Medical Assistance
A type of health insurance plan. - Provincial Medical Assistance
A type of health insurance plan.
Domain Diagram Legend
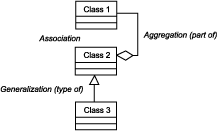
- A reporting facility creates an event report, which consists of patient, tumor, and treatment or service data. Types of event reports include rapid, updated, corrected, and routine event reports (abstracts).
- The event report contributes to a cancer case (consolidated record) that consists of summarized patient, tumor, treatment, follow-up, and stage data.
- The event report is sent to a central cancer registry (CCR).
- A central registry registrar works with a reporting facility and works for a CCR.
- A central registry registrar uses the cancer case (consolidated record), which is contained in the CCR.
- The CCR provides cancer information; reports to CDC’s National Program of Cancer Registries, NCI’s Surveillance, Epidemiology and End Results (SEER) Program, and the North American Association of Central Cancer Registries (NAACCR); and provides data for research.
Healthcare (hospital) facility data sources include hospitals, independent healthcare practitioners, radiation oncology centers, freestanding surgery centers, freestanding pathology laboratories, nursing homes, Indian Health Services (IHS) and local tribe clinics, prisons, and Offices of the Medical Examiner
Hospital data sources include the hospital registry, oncology clinics, hospital pathology departments, claims (Medicare, Medicaid, private insurance, and billing), the hospital disease index, e-Prescriptions, and diagnostic imaging departments.
Non-healthcare (non-hospital) facility data sources include health insurance plans, state cancer registries, state health departments, the Bureau of Vital Statistics, the National Death Index, the census tract database, voter registration, and the Department of Motor Vehicles (DMV). Health maintenance organizations (HMOs), the Center for Medicare and Medicaid Services, and state and provincial medical assistance plans are types of health insurance plans. Healthcare facility and non-healthcare facility data sources are types of reporting facilities.
Note: Definitions for individual terms can be found in the Glossary.
- Page last reviewed: January 15, 2016
- Page last updated: January 15, 2016
- Content source:
- Maintained By:


 ShareCompartir
ShareCompartir