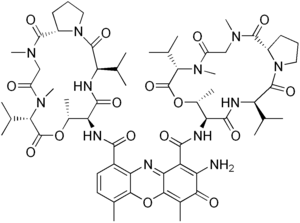
Dactinomycin
 | |
 | |
| Names | |
|---|---|
| Trade names | Cosmegen |
| Other names | Actinomycin D 2-Amino- 4,6-dimethyl- 3-oxo- 3H-phenoxazine- 1,9-dicarboxylic acid bis- [(5,12-diisopropyl- 9,13,16-trimethyl- 4,7,11,14,17-pentaoxo- hexadecahydro- 10-oxa- 3a,6,13,16-tetraaza- cyclopentacyclohexadecen- 8-yl)- amide] |
IUPAC name
| |
| Clinical data | |
| Pregnancy category |
|
| Routes of use | IV |
| Defined daily dose | Not established[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| US NLM | Dactinomycin |
| MedlinePlus | a682224 |
| Legal | |
| Legal status | |
| Pharmacokinetics | |
| Protein binding | 5% |
| Elimination half-life | 36 hours |
| Chemical and physical data | |
| Formula | C62H86N12O16 |
| Molar mass | 1255.438 g·mol−1 |
InChI
| |
Dactinomycin, also known as actinomycin D, is a chemotherapy medication used to treat a number of types of cancer.[2] This includes Wilms tumor, rhabdomyosarcoma, Ewing's sarcoma, trophoblastic neoplasm, testicular cancer, and certain types of ovarian cancer.[2] It is given by injection into a vein.[2]
Most people develop side effects.[2] Common side effects include bone marrow suppression, vomiting, mouth ulcers, hair loss, liver problems, infections, and muscle pains.[2] Other serious side effects include future cancers, allergic reactions, and tissue death at the site of injection.[2] Use in pregnancy may harm the baby.[2] Dactinomycin is in the cytotoxic antibiotic family of medications.[3] It is believed to work by blocking the creation of RNA.[2]
Dactinomycin was approved for medical use in the United States in 1964.[2] It is on the World Health Organization's List of Essential Medicines.[4] The wholesale cost in the developing world is about US$24.73 per 500 mcg vial.[5] It is produced from bacteria Streptomyces parvullus.[2]
Medical use
Actinomycin is a clear, yellowish liquid administered intravenously and most commonly used in treatment of a variety of cancers, including:
- Gestational trophoblastic neoplasia[6]
- Wilms' tumor[7]
- Rhabdomyosarcoma[8]
- Ewing's sarcoma[9]
- Malignant hydatidiform mole[10]
Sometimes it will be combined with other drugs in chemotherapy regimens, like the VAC regimen (with vincristine and cyclophosphamide) for treating rhabdomyosarcoma and Ewing's sarcoma.
It is also used as a radiosensitizer in adjunct to radiotherapies, since it can increase the radiosensitivity of tumor cells by inhibiting repair of sublethal radiation damage and delay the onset of the compensatory hyperplasia that occurs following irradiation.[11]
Dosage
The defined daily dose is not established.[1]
Side effects
Common adverse drug reaction includes bone marrow suppression, fatigue, hair loss, mouth ulcer, loss of appetite and diarrhea. Actinomycin is a vesicant, if extravasation occurs.
Mechanism
In cell biology, actinomycin D is shown to have the ability to inhibit transcription. Actinomycin D does this by binding DNA at the transcription initiation complex and preventing elongation of RNA chain by RNA polymerase.[12]
History
Actinomycin D was the first antibiotic shown to have anti-cancer activity.[13] It was first isolated by Selman Waksman and his co-worker H. Boyd Woodruff in 1940.[14] It was approved by the U.S. Food and Drug Administration (FDA) on December 10, 1964 and launched by Merck Sharp and Dohme under the trade name Cosmegen.
Research use
Because actinomycin can bind DNA duplexes, it can also interfere with DNA replication, although other chemicals such as hydroxyurea are better suited for use in the laboratory as inhibitors of DNA synthesis.
Actinomycin D and its fluorescent derivative, 7-aminoactinomycin D (7-AAD), are used as stains in microscopy and flow cytometry applications. The affinity of these stains/compounds for GC-rich regions of DNA strands makes them excellent markers for DNA. 7-AAD binds to single stranded DNA; therefore it is a useful tool in determining apoptosis and distinguishing between dead cells and live ones.[15]
References
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 28 November 2020. Retrieved 1 September 2020.
- 1 2 3 4 5 6 7 8 9 10 "Dactinomycin". The American Society of Health-System Pharmacists. Archived from the original on 11 September 2017. Retrieved 8 December 2016.
- ↑ British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. p. 582. ISBN 9780857111562.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ "Dactinomycin". International Drug Price Indicator Guide. Archived from the original on 29 March 2019. Retrieved 8 December 2016.
- ↑ Turan T, Karacay O, Tulunay G, Boran N, Koc S, Bozok S, Kose MF (2006). "Results with EMA/CO (etoposide, methotrexate, actinomycin D, cyclophosphamide, vincristine) chemotherapy in gestational trophoblastic neoplasia". International Journal of Gynecological Cancer. 16 (3): 1432–8. doi:10.1111/j.1525-1438.2006.00606.x. PMID 16803542.
- ↑ D'Angio GJ, Evans A, Breslow N, Beckwith B, Bishop H, Farewell V, et al. (May 1981). "The treatment of Wilms' tumor: results of the Second National Wilms' Tumor Study". Cancer. 47 (9): 2302–11. doi:10.1002/1097-0142(19810501)47:9<2302::aid-cncr2820470933>3.0.co;2-k. PMID 6164480.
- ↑ Khatua S, Nair CN, Ghosh K (November 2004). "Immune-mediated thrombocytopenia following dactinomycin therapy in a child with alveolar rhabdomyosarcoma: the unresolved issues". Journal of Pediatric Hematology/Oncology. 26 (11): 777–9. doi:10.1097/00043426-200411000-00020. PMID 15543019.
- ↑ Jaffe N, Paed D, Traggis D, Salian S, Cassady JR (November 1976). "Improved outlook for Ewing's sarcoma with combination chemotherapy (vincristine, actinomycin D and cyclophosphamide) and radiation therapy". Cancer. 38 (5): 1925–30. doi:10.1002/1097-0142(197611)38:5<1925::AID-CNCR2820380510>3.0.CO;2-J. PMID 991106.
- ↑ Uberti EM, Fajardo M, Ferreira SV, Pereira MV, Seger RC, Moreira MA, et al. (December 2009). "Reproductive outcome after discharge of patients with high-risk hydatidiform mole with or without use of one bolus dose of actinomycin D, as prophylactic chemotherapy, during the uterine evacuation of molar pregnancy". Gynecologic Oncology. 115 (3): 476–81. doi:10.1016/j.ygyno.2009.09.012. PMID 19818481.
- ↑ Hagemann RF, Concannon JP (April 1973). "Mechanism of intestinal radiosensitization by actinomycin D". The British Journal of Radiology. 46 (544): 302–8. doi:10.1259/0007-1285-46-544-302. PMID 4720744.
- ↑ Sobell HM (August 1985). "Actinomycin and DNA transcription". Proceedings of the National Academy of Sciences of the United States of America. 82 (16): 5328–31. Bibcode:1985PNAS...82.5328S. doi:10.1073/pnas.82.16.5328. PMC 390561. PMID 2410919.
- ↑ Hollstein U (1974). "Actinomycin. Chemistry and mechanism of action". Chemical Reviews. 74 (6): 625–652. doi:10.1021/cr60292a002.
- ↑ Waksman SA, Woodruff HB (1940). "Bacteriostatic and bacteriocidal substances produced by soil actinomycetes". Proceedings of the Society for Experimental Biology and Medicine. 45: 609–614. doi:10.3181/00379727-45-11768. S2CID 84774334.
- ↑ Toba K, Koike T, Watanabe K, Fuse I, Takahashi M, Hashimoto S, et al. (January 2000). "Cell kinetic study of normal human bone marrow hematopoiesis and acute leukemia using 7AAD/PY". European Journal of Haematology. 64 (1): 10–21. doi:10.1034/j.1600-0609.2000.09005.x. PMID 10680701.
External links
| External sites: |
|
|---|---|
| Identifiers: |