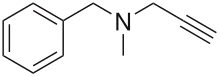
Pargyline
 | |
 | |
| Clinical data | |
|---|---|
| MedlinePlus | a682088 |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.008.275 |
| Chemical and physical data | |
| Formula | C11H13N |
| Molar mass | 159.232 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Pargyline (brand name Eutonyl) is an irreversible selective monoamine oxidase (MAO)-B inhibitor drug (IC50 for MAO-A is 0.01152 μmol/L and for MAO-B is 0.00820 μmol/L)[1][2] It was brought to market in the US and the UK by Abbott in 1963 as an antihypertensive drug branded "Eutonyl". It was one of several MAO inhibitors introduced in the 1960s including nialamide, isocarboxazid, phenelzine, and tranylcypromine.[3]: 146 [4]: 60 [5][6] By 2007 the drug was discontinued[7] and as of 2014 there were no generic versions available in the US.[8] In addition to its actions as an MAOI, pargyline has been found to bind with high affinity to the I2 imidazoline receptor (an allosteric site on the MAO enzyme).[9]
See also
References
- ↑ Fisar Z, Hroudová J, Raboch J (2010). "Inhibition of monoamine oxidase activity by antidepressants and mood stabilizers". Neuro Endocrinol Lett. 31 (5): 645–56. PMID 21200377.
- ↑ Murphy DL, Karoum F, Pickar D, et al. (1998). "Differential trace amine alterations in individuals receiving acetylenic inhibitors of MAO-A (clorgyline) or MAO-B (selegiline and pargyline)". J. Neural Transm. Suppl. Journal of Neural Transmission. Supplement. 52: 39–48. doi:10.1007/978-3-7091-6499-0_5. ISBN 978-3-211-83037-6. PMID 9564606.
- ↑ Edward Shorter, A historical dictionary of psychiatry. Oxford University Press, Inc 2005. ISBN 0195176685
- ↑ William M. Wardell and Louis Lasagna. Regulation Drug Development (Evaluative Studies 21) American Enterprise Institute (1975) ISBN 0844731676
- ↑ Council on Drugs New Drugs and Developments in Therapeutics: Pargyline Hydrochloride (Eutonyl) JAMA. 1963;184(11):887. doi:10.1001/jama.1963.03700240079013.
- ↑ "Eutonyl and MAO inhibitors". Drug and Therapeutics Bulletin. 1 (15): 59–60. 15 November 1963. doi:10.1136/dtb.1.15.59. ISSN 1755-5248. S2CID 220162992.
Pargyline is promoted only for the treatment of hypertension, and not for depression.
- ↑ W. Steven Pray Interactions Between Nonprescription Products and Psychotropic Medications US Pharmacist. 2007;32(11):12-15.
- ↑ FDA Eutonyl in the Drugs@FDA Database Accessed July 19, 2014
- ↑ Critical Reviews in Neurobiology. CRC Press. 1995. p. 43.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.