Gatifloxacin
 | |
 | |
| Names | |
|---|---|
| Trade names | Gatiflo, Tequin, Zymar, others |
IUPAC name
| |
| Clinical data | |
| Drug class | Fluoroquinolone[1] |
| Main uses | Bacterial conjunctivitis[1] |
| Side effects | Eye pain, abnormal taste, redness of the eyes[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | Eye drops, by mouth (discontinued), IV (discontinued)[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a605012 |
| Legal | |
| Legal status |
|
| Pharmacokinetics | |
| Protein binding | 20% |
| Elimination half-life | 7 to 14 hours |
| Chemical and physical data | |
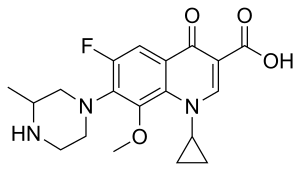
| Formula | C19H22FN3O4 |
| Molar mass | 375.400 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Gatifloxacin, sold under the brand name Tequin among others, is an antibiotic used to treat bacterial conjunctivitis.[1] It is used as an eye drop.[1]
Common side effect effects include eye pain, abnormal taste, and increased redness of the eyes.[1] Other side effects may include allergic reactions.[1] It is in the fluoroquinolone family and works by inhibits the bacterial enzymes DNA gyrase and topoisomerase IV.[1]
Gatifloxacin was patented in 1986 and approved for medical use in 1999.[3] The by mouth and injectable formulations were withdrawn in the United States in 2006 due to the side effects of high or low blood sugar.[2]
Medical use
Dosage
One drop may be used every 2 hours up to 9 times per day on the first day, followed by one drop two to 4 times per day for 6 days.[1]
Side effects
A 2006 study found side effects including dysglycemia.[4] An editorial called for the Food and Drug Administration (FDA) to consider giving Tequin a black box warning.[5] This editorial followed distribution of a letter dated February 15 by Bristol-Myers Squibb to health care providers indicating action taken with the FDA to strengthen warnings for the medication.[6] Subsequently, Bristol-Myers Squibb reported it would stop manufacture of Tequin, end sales of the drug after existing stockpiles were exhausted, and return all rights to Kyorin.[7]
By contrast, ophthalmic gatifloxacin is generally well tolerated. The observed systemic concentration of the drug following oral administration of 400 mg (0.01 ounces) gatifloxacin is approximately 800 times higher than that of the 0.5% gatifloxacin eye drop. Given as an eye drop, gatifloxacin has very low systemic exposure. Therefore, the systemic exposures resulting from the gatifloxacin ophthalmic solution are not likely to pose any risk for systemic toxicities.
Contraindications
Society and culture
Availability
Gatifloxacin is currently available in the US and Canada only as an ophthalmic solution.
In 2011, the Union Health and Family Welfare Ministry of India banned the manufacture, sale, and distribution of gatifloxacin because of its side effects.[8]
In China, gatifloxacin is sold in tablet as well as in eye drop formulations.
Brand names
Bristol-Myers Squibb introduced gatifloxacin in 1999 under the proprietary name Tequin for the treatment of respiratory tract infections, having licensed the medication from Kyorin Pharmaceutical Company of Japan. Allergan produces it in eye-drop formulation under the names Zymar and Zymaxid. In many countries, gatifloxacin is also available as tablets and in various aqueous solutions for intravenous therapy.
References
- 1 2 3 4 5 6 7 8 9 "Gatifloxacin (EENT) Monograph for Professionals". Drugs.com. Archived from the original on 25 November 2020. Retrieved 3 December 2021.
- 1 2 "Determination That TEQUIN (Gatifloxacin) Was Withdrawn From Sale for Reasons of Safety or Effectiveness". Archived from the original on 10 May 2021. Retrieved 3 December 2021.
- ↑ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 501. ISBN 9783527607495. Archived from the original on 2021-10-31. Retrieved 2021-04-01.
- ↑ Park-Wyllie, Laura Y.; David N. Juurlink; Alexander Kopp; Baiju R. Shah; Thérèse A. Stukel; Carmine Stumpo; Linda Dresser; Donald E. Low; Muhammad M. Mamdani (March 2006). "Outpatient Gatifloxacin Therapy and Dysglycemia in Older Adults". The New England Journal of Medicine. 354 (13): 1352–1361. doi:10.1056/NEJMoa055191. hdl:1807/16915. PMID 16510739. Note: publication date 30 March; available on-line 1 March
- ↑ Gurwitz, Jerry H. (March 2006). "Serious Adverse Drug Effects — Seeing the Trees through the Forest". The New England Journal of Medicine. 354 (13): 1413–1415. doi:10.1056/NEJMe068051. PMID 16510740.
- ↑ Lewis-Hall, Freda (February 15, 2006). "Dear Healthcare Provider" (PDF). Bristol-Myers Squibb. Archived (PDF) from the original on March 25, 2006. Retrieved May 1, 2006.
- ↑ Schmid, Randolph E. (May 1, 2006). "Drug Company Taking Tequin Off Market". Associated Press. Archived from the original on November 25, 2007. Retrieved 2006-05-01.
- ↑ "Two drugs banned". The Hindu. Chennai, India. 19 March 2011. Archived from the original on 15 January 2016. Retrieved 1 April 2021.
External links
| Identifiers: |
|---|