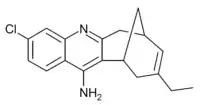
Huprine X
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C18H19ClN2 |
| Molar mass | 298.81 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Huprine X is a synthetic cholinergic compound developed as a hybrid between the natural product Huperzine A and the synthetic drug tacrine. It is one of the most potent reversible inhibitors of acetylcholinesterase known, with a binding affinity of 0.026nM,[1] as well as showing direct agonist activity at both nicotinic and muscarinic acetylcholine receptors.[2][3] In animal studies it has nootropic and neuroprotective effects, and is used in research into Alzheimer's disease,[4][5][6][7] and although huprine X itself has not been researched for medical use in humans, a large family of related derivatives have been developed.[8][9][10]
References
- ↑ Camps P, Cusack B, Mallender WD, El Achab RE, Morral J, Muñoz-Torrero D, Rosenberry TL (February 2000). "Huprine X is a novel high-affinity inhibitor of acetylcholinesterase that is of interest for treatment of Alzheimer's disease". Molecular Pharmacology. 57 (2): 409–17. PMID 10648652.
- ↑ Roman S, Vivas NM, Badia A, Clos MV (June 2002). "Interaction of a new potent anticholinesterasic compound (+/-)huprine X with muscarinic receptors in rat brain". Neuroscience Letters. 325 (2): 103–6. doi:10.1016/s0304-3940(02)00245-8. PMID 12044632. S2CID 30405842.
- ↑ Roman S, Badia A, Camps P, Clos MV (January 2004). "Potentiation effects of (+/-)huprine X, a new acetylcholinesterase inhibitor, on nicotinic receptors in rat cortical synaptosomes". Neuropharmacology. 46 (1): 95–102. doi:10.1016/j.neuropharm.2003.08.005. PMID 14654101. S2CID 19189750.
- ↑ Ratia M, Giménez-Llort L, Camps P, Muñoz-Torrero D, Clos MV, Badia A (June 2010). "Behavioural effects and regulation of PKCalpha and MAPK by huprine X in middle aged mice". Pharmacology, Biochemistry, and Behavior. 95 (4): 485–93. doi:10.1016/j.pbb.2010.03.013. PMID 20363245. S2CID 34525530.
- ↑ Ratia M, Giménez-Llort L, Camps P, Muñoz-Torrero D, Pérez B, Clos MV, Badia A (2013). "Huprine X and huperzine A improve cognition and regulate some neurochemical processes related with Alzheimer's disease in triple transgenic mice (3xTg-AD)". Neuro-Degenerative Diseases. 11 (3): 129–40. doi:10.1159/000336427. PMID 22626981. S2CID 46024586.
- ↑ Giménez-Llort L, Ratia M, Pérez B, Camps P, Muñoz-Torrero D, Badia A, Clos MV (April 2017). "Behavioural effects of novel multitarget anticholinesterasic derivatives in Alzheimer's disease". Behavioural Pharmacology. 28 (2 and 3-Spec Issue): 124–131. doi:10.1097/FBP.0000000000000292. PMID 28125507.
- ↑ Relat J, Pérez B, Camps P, Muñoz-Torrero D, Badia A, Victòria Clos M (January 2018). "Huprine X Attenuates The Neurotoxicity Induced by Kainic Acid, Especially Brain Inflammation". Basic & Clinical Pharmacology & Toxicology. 122 (1): 94–103. doi:10.1111/bcpt.12852. PMID 28724203.
- ↑ Ronco C, Sorin G, Nachon F, Foucault R, Jean L, Romieu A, Renard PY (July 2009). "Synthesis and structure-activity relationship of Huprine derivatives as human acetylcholinesterase inhibitors". Bioorganic & Medicinal Chemistry. 17 (13): 4523–36. doi:10.1016/j.bmc.2009.05.005. PMID 19473849.
- ↑ Ronco C, Foucault R, Gillon E, Bohn P, Nachon F, Jean L, Renard PY (May 2011). "New huprine derivatives functionalized at position 9 as highly potent acetylcholinesterase inhibitors". ChemMedChem. 6 (5): 876–88. doi:10.1002/cmdc.201000523. PMID 21344648. S2CID 10893910.
- ↑ Galdeano C, Viayna E, Sola I, Formosa X, Camps P, Badia A, et al. (January 2012). "Huprine-tacrine heterodimers as anti-amyloidogenic compounds of potential interest against Alzheimer's and prion diseases". Journal of Medicinal Chemistry. 55 (2): 661–9. doi:10.1021/jm200840c. PMID 22185619.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.