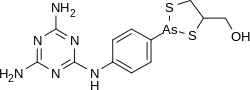
Melarsoprol
 | |
 | |
| Names | |
|---|---|
| Trade names | Arsobal[1] |
| Other names | Mel B, Melarsen Oxide-BAL[2] |
IUPAC name
| |
| Clinical data | |
| Main uses | African trypanosomiasis[3] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | IV |
| Defined daily dose | 60 mg[4] |
| External links | |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Pharmacokinetics | |
| Elimination half-life | 35 hours |
| Excretion | Kidney |
| Chemical and physical data | |
| Formula | C12H15AsN6OS2 |
| Molar mass | 398.33 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Melarsoprol is a medication used for the treatment of sleeping sickness (African trypanosomiasis).[1] It is specifically used for second-stage disease caused by Trypanosoma brucei rhodesiense when the central nervous system is involved.[1] For Trypanosoma brucei gambiense, the medication eflornithine is usually preferred.[1] It is effective in about 95% of people.[5] It is given by injection into a vein.[2]
Melarsoprol has a high number of side effects.[6] Common side effects include brain dysfunction, numbness, rashes, and kidney and liver problems.[2] About 1-5% of people die during treatment.[5] In those with glucose-6-phosphate dehydrogenase (G6PD) deficiency, red blood cell breakdown may occur.[2] It has not been studied in pregnancy and such use is not recommended.[2][3] It works by blocking pyruvate kinase, an enzyme required for the parasite to make adenosine triphosphate.[2]
Melarsoprol has been used medically since 1949.[1] It is on the World Health Organization's List of Essential Medicines.[7] In regions of the world where the disease is common, melarsoprol is provided for free by the World Health Organization.[6] It is not commercially available in Canada or the United States.[2] In the United States, it may be obtained from the Centers for Disease Control and Prevention, while in Canada it is available from Health Canada.[1][2]
Medical uses
People diagnosed with trypanosome-caused disease should be treated with an anti-trypanosomal. Treatment is based on stage, 1 or 2, and parasite,T. b. rhodesiense or T. b. gambiense. In stage 1 disease, trypanosomes are present only in the peripheral circulation. In stage 2 disease, trypanosomes have crossed the blood brain barrier and are present in the central nervous system.[8]
The following are considerable treatment options:[8]
Melarsoprol is a treatment used during the second stage of the disease. So far, it is the only treatment available for late-stage T. b. rhodesiense.[9]
Due to high toxicity, melarsoprol is reserved only for the most dangerous cases. Other agents associated with lower toxicity levels are used during stage 1 of the disease.[10] The approval of the nifurtimox-eflornithine combination therapy (NECT) in 2009 for the treatment of T. b. gambiense limited the use of melarsoprol in the treatment of second-stage T. b. rhodesiense.[11]
Failure rates of 27% in certain African countries have been reported.[12] This was caused by both drug resistance and additional mechanisms that have not yet been elucidated. Resistance is likely due to transport problems associated with the P2 transporter, an adenine-adenosine transporter. Resistance can occur with point mutations within this transporter.[13] Resistance has been present since the 1970s.[14]
Dosage
The defined daily dose is 60 mg by injection.[4] It is generally given at a dose of 2.2 mg/kg to a maximum of 180 mg once per day for 10 days.[3]
Side effects
Although melarsoprol cures about 96% of people with late stage disease, its toxicity limits its use.[9] About 1-5% of people die during treatment.[5] As a toxic organic compound of arsenic, melarsoprol is a dangerous treatment that is typically only administered by injection under the supervision of a licensed physician. Notable side effects are similar to arsenic poisoning. Among clinicians, it is colloquially referred to as "arsenic in antifreeze".[15] Severe and life-threatening adverse reactions are associated with melarsoprol. It is known to cause a range of side effects including convulsions, fever, loss of consciousness, rashes, bloody stools, nausea and vomiting. In approximately 5-10% of cases, it causes encephalopathy. Of those, about 50% die due to encephalopathy-related adverse reactions.[8] Additional potentially serious side effects of melarsoprol include damage to the heart, presence of albumin in the urine that could be associated with kidney damage, and an increase in blood pressure.[5]
Cautions
Numerous warnings must be examined before melarsoprol treatment can be initiated. Prior to initiation, the following must be noted: glucose-6-phosphate dehydrogenase deficiency, kidney or liver disease, cardiac problems (high blood pressure, irregular beating of the heart or arrhythmias, any damage to the heart muscles and potential signs of heart failure), preexisting nervous system disorders, and any signs of leprosy.
Routine laboratory testing is needed before and after melarsoprol initiation. Laboratory parameters for both therapeutic effects and toxic effects need to be evaluated.
Blood analysis is used to detect the presence of trypanosomes. An evaluation of the cerebrospinal fluid via a lumbar puncture is also used to determine an individual's white blood count and level of protein. These are diagnostic criteria such that the presence of trypanosomes, an elevated white blood count greater than five per microliter, or a protein content greater than 40 mg are considered abnormal and initiation should be considered. Continuous cerebrospinal fluid evaluation should be repeated every six months for at least three years in individuals that have undergone melarsoprol treatment.
To assess potential concerns related to toxicity, the following should be completed: a complete blood count, an assessment of electrolyte levels, liver and kidney function tests, and a urinalysis to detect the appearance, concentration and content of the urine.
Melarsoprol should be given using glass syringes (if they can be reliably sterilised). The propylene glycol it contains is capable of dissolving plastic.[16]
Pregnancy and breastfeeding
As of 2020, melarsoprol is not recommended for use in pregnant women.[3] The World Health Organization suggests that treatment be deferred until immediately after delivery since the effects of the medication on the developing fetus have not yet been established.[5]
Lactation guidelines associated with melarsoprol have not yet been established.
Mechanism of action
Melarsoprol is a prodrug, which is metabolized to melarsen oxide (Mel Ox) as its active form. Mel Ox is an phenylarsonous acid derivative that irreversibly binds to sulfhydryl groups on pyruvate kinase, which disrupts energy production in the parasite. The inability to distinguish between host and parasite PK renders this drug highly toxic with many side effects.
Mel Ox also reacts with trypanothione (a spermidine-glutathione adduct that replaces glutathione in trypanosomes). It forms a melarsen oxide-trypanothione adduct (Mel T) that competitively inhibits trypanothione reductase, effectively killing the protist.[13]
Dosage
Two arsenic-containing stereoisomers exist in a 3:1 molar ratio. Since melarsoprol is insoluble in water, dosage occurs via a 3.6% propylene glycol intravenous injection.[13] To avoid the risk of injection site reactions, melarsoprol must be given slowly.
Melarsoprol used for the treatment of African trypanosomiasis with CNS involvement is given under a complicated dosing schedule. The dosing schedule for children and adults is 2–3.6 mg/kg/day intravenously for three days, then repeated every seven days for a total of three series.[8] To monitor for relapse, follow-up is recommended every six months for at least two years.[5]
Pharmacokinetics
The half-life of melarsoprol is less than one hour, but bioassays indicate a 35-hour half-life. This is commonly associated with pharmacologic agents that have active metabolites. One such metabolite, Mel Ox, reaches maximum plasma levels about 15 minutes after melarsoprol injection. Melarsoprol clearance is 21.5 ml/min/kg and the Mel Ox half-life is approximately 3.9 hours.[17]
Society and culture
Melarsoprol is produced by Sanofi-Aventis and under an agreement with the WHO, they donate melarsoprol to countries where the disease is common.
Melarsoprol was used to treat a patient with African trypanosomiasis on season 1 episode 7 'Fidelity' on the medical drama House MD.[18]
References
- 1 2 3 4 5 6 "Our Formulary Infectious Diseases Laboratories CDC". www.cdc.gov. 22 September 2016. Archived from the original on 16 December 2016. Retrieved 7 December 2016.
- 1 2 3 4 5 6 7 8 "Melarsoprol Drug Information, Professional". www.drugs.com. 20 December 1994. Archived from the original on 30 December 2016. Retrieved 7 December 2016.
- 1 2 3 4 "MELARSOPROL injectable - Essential drugs". medicalguidelines.msf.org. Archived from the original on 6 May 2021. Retrieved 2 September 2020.
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 22 January 2021. Retrieved 2 September 2020.
- 1 2 3 4 5 6 "WHO Model Prescribing Information: Drugs Used in Parasitic Diseases - Second Edition: Protozoa: African trypanosomiasis: Melarsoprol". WHO. 1995. Archived from the original on 2016-11-10. Retrieved 2016-11-09.
- 1 2 "Trypanosomiasis, human African (sleeping sickness)". World Health Organization. February 2016. Archived from the original on 4 December 2016. Retrieved 7 December 2016.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- 1 2 3 4 CDC (2013). "Disease Control and Prevention: Parasites – African Trypanosomiasis". Archived from the original on 2017-06-19.
{{cite journal}}: Cite journal requires|journal=(help) - 1 2 "Trypanosoma brucei gambiense and Trypanosoma brucei rhodesiense (African Trypanosomiasis) - Infectious Disease and Antimicrobial Agents". www.antimicrobe.org. Archived from the original on 2016-11-28. Retrieved 2016-11-17.
- ↑ Bisser S; N'Siesi FX; Lejon V; et al. (2007). "Equivalence trial of melarsoprol and nifurtimox monotherapy and combination therapy for the treatment of second-stage Trypanosoma brucei rhodesiense sleeping sickness". J. Infect. Dis. 195 (3): 322–9. doi:10.1086/510534. PMID 17205469.
- ↑ Farrar J (2014). "Manson's Tropical Diseases: Expert Consult-Online". 23: 616.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ Kioy, D.; Jannin, J.; Mattock, N. (March 2004). "Human African trypanosomiasis". Nature Reviews Microbiology. 2 (3): 186–187. doi:10.1038/nrmicro848. PMID 15751187.
- 1 2 3 Brunton L (2011). "Goodman & Gillman's The Pharmacological Basis of Therapeutics". McGraw Hill Medical: 1427–28.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ Brun, Reto; Schumacher, Reto; Schmid, Cecile; Kunz, Christina; Burri, Christian (November 2001). "The phenomenon of treatment failures in Human African Trypanosomiasis". Tropical Medicine and International Health. 6 (11): 906–914. doi:10.1046/j.1365-3156.2001.00775.x.
- ↑ Hollingham R (2005). "Curing diseases modern medicine has left behind". New Scientist. 2005 (2482): 40–41. Archived from the original on 2015-05-11.
- ↑ "MELARSOPROL injectable - Essential drugs". medicalguidelines.msf.org. Archived from the original on 6 May 2021. Retrieved 6 December 2019.
- ↑ Keiser J.; Ericsson O; Burri C (2000). "Investigations of the metabolites of the trypanocidal drug melarsoprol". Clinical Pharmacology. 67 (5): 478–88. doi:10.1067/mcp.2000.105990. PMID 10824626.
- ↑ Holtz, Andrew (2006). The Medical Science of House, M.D. Penguin. p. 272. ISBN 1440628734. Archived from the original on 17 April 2020. Retrieved 25 March 2020.
External links
| External sites: |
|
|---|---|
| Identifiers: |