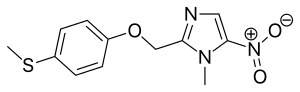
Fexinidazole
 | |
| Names | |
|---|---|
| Other names |
|
IUPAC name
| |
| Clinical data | |
| Main uses | African trypanosomiasis (sleeping sickness) cause by Trypanosoma brucei gambiense[1] |
| Side effects | Nausea, vomiting, headache, trouble sleeping[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Defined daily dose | 1.4 gram[3] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Chemical and physical data | |
| Formula | C12H13N3O3S |
| Molar mass | 279.31 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
Fexinidazole is a medication used to treat African trypanosomiasis (sleeping sickness) cause by Trypanosoma brucei gambiense.[1] It is effective against both first and second stage disease.[1] Some evidence also supports its use in Chagas disease.[4] It is taken by mouth.[4]
Common side effects include nausea, vomiting, headache, and trouble sleeping.[2] Other side effects may include QT prolongation, psychosis, and low white blood cells.[5] It is unclear if use during pregnancy or breast feeding is safe.[5] Fexinidazole is in the antiparasitic and the nitroimidazole family of medications.[4] It is believed to work by turning on certain enzymes within the parasites that result in their death.[2]
Fexinidazole was first described in 1978.[6] It was given a positive opinion by the European Medicines Agency in 2018 and approved for medical use in the United States in 2021.[2][7] It is on the World Health Organization's List of Essential Medicines.[8] Development for sleeping sickness was funded by the Drugs for Neglected Diseases initiative in collaboration with Sanofi.[9]
Medical use
Sleeping sickness
A trial in Africa found fexinidazole to be 91% effective at treating sleeping sickness.[2][10] Though less effective than nifurtimox with eflornithine in severe disease, fexinidazole has the benefit that it can be taken by mouth.[2]
Fexinidazole is the first drug candidate for the treatment of advanced-stage sleeping sickness in thirty years.[11]
Other
It has activity against Trypanosoma cruzi, Tritrichomonas foetus, Trichomonas vaginalis, Entamoeba histolytica,[12] and Trypanosoma brucei.[13] It has not been found to be useful for visceral leishmaniasis.[4]
Dosage
The defined daily dose is 1.4 gram.[3]
Mechanism of action
The biologically relevant active metabolites in vivo are the sulfoxide and sulfone.[14][15]
History
Fexinidazole was discovered by the German pharmaceutical company Hoechst AG, but its development as a pharmaceutical was halted in the 1980s.[16]
Society and culture
Fexinidazole Winthrop, a Sanofi-Aventis product developed with the Drugs for Neglected Diseases Initiative (DNDi), received a positive endorsement from the European Medicines Agency in 2018, for use in non-European markets.[17][18] It was approved for the treatment of Trypanosoma brucei gambiense human African trypanosomiasis (HAT) in the Democratic Republic of the Congo (DRC) in December 2018.[19]
References
- 1 2 3 DIMITROVA, Elena Kostadinova (22 January 2019). "Fexinidazole Winthrop H-W-2320". European Medicines Agency. Archived from the original on 18 January 2021. Retrieved 12 November 2019.
- 1 2 3 4 5 6 "Fexinidazole Winthrop (fexinidazole)" (PDF). EMA. Archived from the original (PDF) on 2 January 2021. Retrieved 12 November 2019.
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 28 August 2021. Retrieved 10 September 2020.
- 1 2 3 4 Deeks, ED (February 2019). "Fexinidazole: First Global Approval". Drugs. 79 (2): 215–220. doi:10.1007/s40265-019-1051-6. PMID 30635838.
- 1 2 "Fexinidazole Winthrop" (PDF). EMA. Archived from the original (PDF) on 12 November 2019. Retrieved 12 November 2019.
- ↑ Gil, Carmen; Rivas, Luis (2017). Drug Discovery for Leishmaniasis. Royal Society of Chemistry. p. 30. ISBN 9781788012584. Archived from the original on 28 August 2021. Retrieved 12 November 2019.
- ↑ Research, Center for Drug Evaluation and (21 June 2022). "Novel Drug Approvals for 2021". FDA. Archived from the original on 3 March 2022. Retrieved 28 October 2022.
- ↑ "World Health Organization model list of essential medicines: 21st list 2019". 2019. hdl:10665/325771.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ "Fexinidazole – DNDi". www.dndi.org. Archived from the original on 29 April 2020. Retrieved 12 November 2019.
- ↑ Mesu VK, Kalonji WM, Bardonneau C, et al. (4 November 2017). "Oral fexinidazole for late-stage African Trypanosoma brucei gambiense trypanosomiasis: a pivotal multicentre, randomised, non-inferiority trial". Lancet. 391 (10116): 144–154. doi:10.1016/s0140-6736(17)32758-7. ISSN 0140-6736. PMID 29113731.
- ↑ Torreele, E; Bourdin Trunz, B; Tweats, D; Kaiser, M; Brun, R; Mazué, G; Bray, MA; Pécoul, B (2010). Boelaert, Marleen (ed.). "Fexinidazole--a new oral nitroimidazole drug candidate entering clinical development for the treatment of sleeping sickness". PLOS Neglected Tropical Diseases. 4 (12): e923. doi:10.1371/journal.pntd.0000923. PMC 3006138. PMID 21200426.
- ↑ Raether, W; Seidenath, H (1983). "The activity of fexinidazole (HOE 239) against experimental infections with Trypanosoma cruzi, trichomonads and Entamoeba histolytica". Annals of Tropical Medicine and Parasitology. 77 (1): 13–26. PMID 6411009.
- ↑ Jennings, FW; Urquhart, GM (1983). "The use of the 2 substituted 5-nitroimidazole, Fexinidazole (Hoe 239) in the treatment of chronic T. brucei infections in mice". Zeitschrift für Parasitenkunde. 69 (5): 577–581. doi:10.1007/bf00926669. PMID 6636983.
- ↑ Wyllie S, Patterson S, Stojanovski L, et al. (2012). "The anti-trypanosome drug fexinidazole shows potential for treating visceral leishmaniasis". Science Translational Medicine. 4 (119): 119re1. doi:10.1126/scitranslmed.3003326. PMC 3457684. PMID 22301556.
- ↑ Sokolova AY, Wyllie S, Patterson S, et al. (2010). "Cross-resistance to nitro drugs and implications for treatment of human African trypanosomiasis". Antimicrobial Agents and Chemotherapy. 54 (7): 2893–900. doi:10.1128/AAC.00332-10. PMC 2897277. PMID 20439607.
- ↑ McNeil, Jr., Donald (8 January 2008). "Jump-Start on Slow Trek to Treatment for a Disease". The New York Times. Archived from the original on 29 July 2020. Retrieved 21 February 2017.
- ↑ "CHMP Summary of Opinion - Fexinidazole Winthrop" (PDF). Archived (PDF) from the original on 24 December 2018. Retrieved 19 November 2018.
- ↑ McNeil, Jr., Donald (16 November 2018). "Rapid Cure Approved for Sleeping Sickness, a Horrific Illness". The New York Times. Archived from the original on 19 November 2018. Retrieved 20 November 2018.
- ↑ "Fexinidazole, the first all-oral treatment for sleeping sickness, approved in Democratic Republic of Congo". Drugs for Neglected Diseases Initiative (DNDi). Archived from the original on 14 March 2020. Retrieved 4 June 2019.
External links
| External sites: |
|
|---|---|
| Identifiers: |
- Package information Archived 12 November 2019 at the Wayback Machine