Tezepelumab
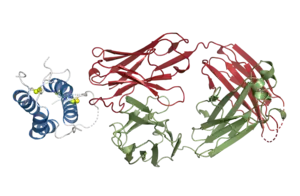 Structural basis for inhibition of TSLP-signaling by Tezepelumab (PDB 5J13)[1] | |
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Human |
| Target | thymic stromal lymphopoietin (TSLP) |
| Names | |
| Trade names | Tezspire |
| Other names | MEDI9929, AMG 157, tezepelumab-ekko |
| Clinical data | |
| Main uses | Asthma[2] |
| Side effects | Joint pain, sore throat[3] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | Subcutaneous |
| Typical dose | 210 mg[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data |
|
| Legal status | |
| Chemical and physical data | |
| Formula | C6400H9844N1732O1992S52 |
| Molar mass | 144590.40 g·mol−1 |
Tezepelumab, sold under the brand name Tezspire, is a medication used for the treatment of asthma.[2][3] Specifically it is used for severe asthma, which is not controlled by other medication, in those at least 12 years old.[3] It is given by injection under the skin every 4 weeks.[3]
The most common side effects include joint pain and sore throat.[3] Other side effects may include allergic reactions.[2] While there is no evidence of harm in pregnancy, such use has not been well studied.[2] It is a monoclonal antibody that blocks thymic stromal lymphopoietin (TSLP), resulting in less airway inflammation.[2][3]
Tezepelumab was approved for medical use in the United States in 2021 and Europe in 2022.[2][3] In the United States it costs about 48,000 USD per year as of 2022.[6] While approved in the UK it was not yet marketed as of 2022.[6]
Medical uses
Tezepelumab is used for the add-on maintenance treatment of people aged twelve years and older with severe asthma.[2][3] In decreases the risk of flares from about 2 to 1 per year.[3]
Dosage
The typical dose is 210 mg every 4 weeks.[2]
History
Two main studies including over 1,500 adults and adolescents with inadequately controlled asthma showed that tezepelumab was effective in reducing the number of severe asthma flare‑ups.[3]
Society and culture
Legal status
On 21 July 2022, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Tezspire, intended as add-on treatment in adult and adolescent patients with severe asthma.[3][7] The applicant for this medicinal product is AstraZeneca AB.[7] Tezepelumab was approved for medical use in the European Union in September 2022.[3]
Research
It is being studied for the treatment of chronic obstructive pulmonary disease, chronic rhinosinusitis with nasal polyps, chronic spontaneous urticaria and eosinophilic esophagitis (EoE).[5]
In phase III trials, tezepelumab demonstrated efficacy compared to placebo for patients with severe, uncontrolled asthma.[8][9]
Structural studies by X-ray crystallography showed that tezepelumab competes against a critical part of the TSLPR binding site on TSLP.[1]
References
- 1 2 Verstraete K, Peelman F, Braun H, Lopez J, Van Rompaey D, Dansercoer A, et al. (April 2017). "Structure and antagonism of the receptor complex mediated by human TSLP in allergy and asthma". Nature Communications. 8 (1): 14937. Bibcode:2017NatCo...814937V. doi:10.1038/ncomms14937. PMC 5382266. PMID 28368013.
- 1 2 3 4 5 6 7 8 9 10 "Tezspire- tezepelumab-ekko injection, solution". DailyMed. Archived from the original on 25 December 2021. Retrieved 24 December 2021.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 "Tezspire EPAR". European Medicines Agency. 19 July 2022. Archived from the original on 22 September 2022. Retrieved 21 September 2022. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "Tezspire Product information". Health Canada. 25 April 2012. Archived from the original on 1 October 2022. Retrieved 1 October 2022.
- 1 2 "Tezspire (tezepelumab) approved in the US for severe asthma". AstraZeneca (Press release). 17 December 2021. Archived from the original on 18 December 2021. Retrieved 17 December 2021.
- 1 2 "Tezepelumab". SPS - Specialist Pharmacy Service. 16 March 2018. Archived from the original on 6 March 2022. Retrieved 24 October 2022.
- 1 2 "Tezspire: Pending EC decision". European Medicines Agency. 21 July 2022. Archived from the original on 28 July 2022. Retrieved 30 July 2022. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "Studies found for: Tezepelumab". ClinicalTrials.Gov. National Library of Medicine, National Institutes of Health, U.S. Department of Health and Human Services. Archived from the original on 30 July 2022. Retrieved 14 March 2020.
- ↑ Menzies-Gow A, Corren J, Bourdin A, Chupp G, Israel E, Wechsler ME, et al. (May 2021). "Tezepelumab in Adults and Adolescents with Severe, Uncontrolled Asthma". New England Journal of Medicine. 384 (19): 1800–09. doi:10.1056/NEJMoa2034975. PMID 33979488. S2CID 234484931. Archived from the original on 28 May 2022. Retrieved 29 March 2022.
External links
| External sites: |
|
|---|---|
| Identifiers: |