wikiHow is a “wiki,” similar to Wikipedia, which means that many of our articles are co-written by multiple authors. To create this article, 19 people, some anonymous, worked to edit and improve it over time.
This article has been viewed 307,083 times.
Learn more...
In many atoms, each electron is said to experience less than the actual nuclear charge because of shielding or screening by the other electrons. For each electron in an atom, Slater's rules provide a value for the screening constant, denoted by σ.
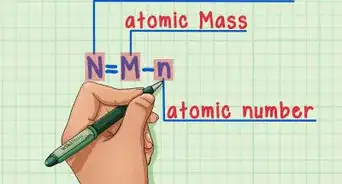
The effective nuclear charge may be defined as the actual nuclear charge (Z) minus the screening effect caused by the electrons intervening between the nucleus and valence electron.[1]
Effective nuclear charge, Z* = Z - σ Where, Z= Atomic number, σ = Shielding or screening constant.
To calculate the effective nuclear charge (Z*) we need the value of screening constant (σ) which can be calculated by using following rules.
Steps
-
1Write down the electronic configuration of the element as shown below.[2]
- (1s) (2s, 2p) (3s, 3p) (3d) (4s, 4p) (4d) (4f) (5s, 5p) (5d)…
- Fill the electrons according to Aufbau principle.
- Any electrons to the right of the electron of interest do not contribute to shielding constant.
- The shielding constant for each group is formed as the sum of the following contributions:
- Each other electron in the same group as the electron of interest shield to an extent of 0.35 nuclear charge units except 1s group, in which the other electron contributes only 0.30.
- If the group (n) is of [s, p] type, an amount of 0.85 from each electron in (n-1)th shell and an amount of 1.00 for each electron from (n-2) and lower shells is added to the shielding constant.
- If the group is of [d] or [f] type, an amount of 1.00 for each electron from all lying left to that orbital.
-
2For example: (a) Calculate effective nuclear charge in Nitrogen for 2p electron.[3]
- Electronic configuration- (1s2) (2s2, 2p3).
- Screening constant, σ = (0.35 × 4) + (0.85 × 2) = 3.10
- Effective nuclear charge, Z* = Z – σ = 7 – 3.10 = 3.90
Advertisement -
3(b) Calculate effective nuclear charge and screening constant seen in 3p electron in Silicon.[4]
- Electronic configuration- (1s2) (2s2, 2p6)(3s2, 3p2).
- σ = (0.35 × 3) + (0.85 × 8) + (1 × 2) = 9.85
- Z* = Z – σ = 14 – 9.85 = 4.15
-
4(c) Calculate effective nuclear charge in Zinc for 4s electron & for 3d electron.[5]
- Electronic configuration- (1s2) (2s2, 2p6)(3s2, 3p6)(3d10)(4s2).
- For 4s electron,
- σ = (0.35 × 1) + (0.85 × 18) + (1 × 10) = 25.65
- Z* = Z – σ = 30 – 25.65 = 4.35
- For 3d electron,
- σ = (0.35 × 9) + (1 × 18) = 21.15
- Z* = Z – σ = 30 – 21.15 = 8.85
-
5(d) Calculate effective nuclear charge on one of 6s electron in tungsten. (At. No. =74)
- Electronic configuration- (1s2) (2s2, 2p6)(3s2, 3p6)(4s2, 4p6) (3d10) (4f14) (5s2, 5p6)(5d4), (6s2)
- σ = (0.35 × 1) + (0.85 × 12) + (1 × 60) = 70.55
- Z* = Z – σ = 74 – 70.55 =3.45
Community Q&A
-
QuestionHow do I determine the effective nuclear charge for 2p in sodium?
 Community AnswerThe effective nuclear charge for any subshell is the total positive charge of the nucleus minus the total negative charge of the previous subshells. Ffor example, the effective nuclear charge on the 2p orbital in sodium would be 7, because the total nuclear charge is 11, but the 4 electrons in the 1s and 2s orbitals screen 4 lead to an effective nuclear charge of 7.
Community AnswerThe effective nuclear charge for any subshell is the total positive charge of the nucleus minus the total negative charge of the previous subshells. Ffor example, the effective nuclear charge on the 2p orbital in sodium would be 7, because the total nuclear charge is 11, but the 4 electrons in the 1s and 2s orbitals screen 4 lead to an effective nuclear charge of 7. -
QuestionIs effective nuclear charge the same for all of the electrons present in an atom?
 Community AnswerNo. The inner electrons have less shielding while the outer electrons have more shielding, and hence the effective nuclear charge differs from orbit to orbit. However, all electrons in the same orbit have the same effective nuclear charge.
Community AnswerNo. The inner electrons have less shielding while the outer electrons have more shielding, and hence the effective nuclear charge differs from orbit to orbit. However, all electrons in the same orbit have the same effective nuclear charge. -
QuestionWhat is screening constant of the 4s electron of potassium?
 Community AnswerAtomic number = 19. Screening effect of 4s = 0×0.35+8×0.85+10×1 = 0+6.8+10= 16.8. As 4s electron has only 1 electron, so 0×0.35. Then 3s3p has 8 electrons, so 8×0.85. 1s 2s2p has 10 electrons, so 10×1. Now these three are added.
Community AnswerAtomic number = 19. Screening effect of 4s = 0×0.35+8×0.85+10×1 = 0+6.8+10= 16.8. As 4s electron has only 1 electron, so 0×0.35. Then 3s3p has 8 electrons, so 8×0.85. 1s 2s2p has 10 electrons, so 10×1. Now these three are added.
Warnings
- Though these rules may appear confusing, writing the proper electronic configuration will help ensure success.⧼thumbs_response⧽
References
- ↑ https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_Chemistry_-_The_Central_Science_(Brown_et_al.)/07%3A_Periodic_Properties_of_the_Elements/7.02%3A_Shielding_and_Effective_Nuclear_Charge
- ↑ https://www.wou.edu/las/physci/ch412/Periodic%20trends/periodic_trends.htm
- ↑ https://chem.libretexts.org/Courses/Mount_Royal_University/Chem_1201/Unit_2._Periodic_Properties_of_the_Elements/2.06%3A_Slater's_Rules
- ↑ https://www.lamar.edu/arts-sciences/_files/documents/chemistry-biochemistry/dorris/chapter7.pdf
- ↑ https://chem.libretexts.org/Courses/Saint_Marys_College_Notre_Dame_IN/CHEM_342%3A_Bio-inorganic_Chemistry/Readings/Week_1%3A_Analysis_of_Periodic_Trends/1.1%3A_Concepts_and_principles_that_explain_periodic_trends/1.1.2%3A_Effective_Nuclear_Charge