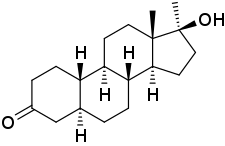
5α-Dihydronormethandrone
5α-Dihydronormethandrone (5α-DHNMT; developmental code name RU-575), also known as 17α-methyl-4,5α-dihydro-19-nortestosterone or as 17α-methyl-5α-estran-17β-ol-3-one, is an androgen/anabolic steroid and a likely metabolite of normethandrone formed by 5α-reductase.[1][2] Analogously to nandrolone and its 5α-reduced metabolite 5α-dihydronandrolone, 5α-DHNMT shows reduced affinity for the androgen receptor relative to normethandrone.[1] Its affinity for the androgen receptor is specifically about 33 to 60% of that of normethandrone.[1]
| Compound | PRTooltip Progesterone receptor | ARTooltip Androgen receptor | ERTooltip Estrogen receptor | GRTooltip Glucocorticoid receptor | MRTooltip Mineralocorticoid receptor | SHBGTooltip Sex hormone-binding globulin | CBGTooltip Corticosteroid binding globulin |
|---|---|---|---|---|---|---|---|
| Normethandrone | 75–125 | 125–150 | <1 | 1–5 | <1 | ? | ? |
| 5α-Dihydronormethandrone | 15–25 | 50–75 | ? | <1 | ? | ? | ? |
| Notes: Values are percentages (%). Reference ligands (100%) were progesterone for the PRTooltip progesterone receptor, testosterone for the ARTooltip androgen receptor, estradiol for the ERTooltip estrogen receptor, dexamethasone for the GRTooltip glucocorticoid receptor, and aldosterone for the MRTooltip mineralocorticoid receptor. Sources: See template. | |||||||
 | |
| Clinical data | |
|---|---|
| Other names | 5α-DHNMT; RU-575; 17α-Methyl-4,5α-dihydro-19-nortestosterone; 17α-Methyl-5α-estran-17β-ol-3-one |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| Chemical and physical data | |
| Formula | C19H30O2 |
| Molar mass | 290.447 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
See also
References
- Ojasoo T, Delettré J, Mornon JP, Turpin-VanDycke C, Raynaud JP (1987). "Towards the mapping of the progesterone and androgen receptors". J. Steroid Biochem. 27 (1–3): 255–69. doi:10.1016/0022-4731(87)90317-7. PMID 3695484.
- Schjølberg, T. H. (2013). In Vitro Synthesis of Metabolites of three Anabolic Androgenic Steroids, by Human Liver Microsomes (Master's thesis, Institutt for bioteknologi). https://brage.bibsys.no/xmlui/handle/11250/246018
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.