Firocoxib
Firocoxib sold under the brand names Equioxx and Previcox among others, is a nonsteroidal anti-inflammatory drug of the COX-2 inhibitor (coxib) class, approved for use in horses (Equioxx) and for use in dogs (Previcox).[1][3] Firocoxib was the first COX-2 inhibitor approved by the U.S. Food and Drug Administration for horses.[7] Firocoxib is not intended or approved for use in human medicine.[1][3][4][5][8]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Equioxx, Previcox |
| AHFS/Drugs.com | Veterinary Use Veterinary Use |
| License data | |
| Routes of administration | By mouth |
| ATCvet code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
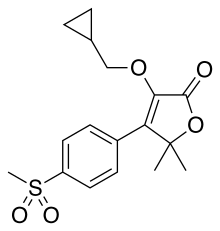
| Formula | C17H20O5S |
| Molar mass | 336.40 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Firocoxib manufactured by Merial was approved for veterinary use in the United States for dogs in July 2004,[9] and for horses in July 2007 as an oral paste (EQUIOXX) and July 2016 as tablets.[10][11]
Firocoxib is also available as a generic medication for horses[11] and for dogs.[9]
References
- "Equioxx- firocoxib tablet, chewable". DailyMed. 14 July 2020. Archived from the original on 3 August 2022. Retrieved 2 August 2022.
- "Equioxx- firocoxib paste". DailyMed. 24 February 2022. Archived from the original on 3 August 2022. Retrieved 2 August 2022.
- "Previcox- firocoxib tablet, chewable". DailyMed. 23 July 2020. Archived from the original on 3 August 2022. Retrieved 2 August 2022.
- "Equioxx EPAR". European Medicines Agency. Archived from the original on 25 April 2022. Retrieved 2 August 2022.
- "Previcox EPAR". European Medicines Agency. Archived from the original on 31 July 2021. Retrieved 2 August 2022.
- "New NSAID Equioxx (firocoxib) Approved by USEF". The Horse. 2 July 2007. Archived from the original on 18 May 2011. Retrieved 18 April 2008.
- Equioxx Archived 21 December 2014 at the Wayback Machine, European Medicines Agency
- "FDA Approves the First Generic Firocoxib Tablets for Dogs". U.S. Food and Drug Administration. 7 June 2022. Archived from the original on 3 August 2022. Retrieved 2 August 2022.
- "Merial launches first firocoxib for horses". DVM 360. 1 July 2007. Retrieved 3 October 2023.
- "FDA Approves the First Generic Firocoxib Tablets for Horses". U.S. Food and Drug Administration (FDA). 1 August 2022. Archived from the original on 3 August 2022. Retrieved 2 August 2022.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.