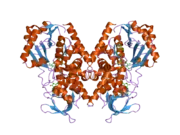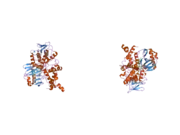Monoamine oxidase A
Monoamine oxidase A, also known as MAO-A, is an enzyme (E.C. 1.4.3.4) that in humans is encoded by the MAOA gene.[5][6] This gene is one of two neighboring gene family members that encode mitochondrial enzymes which catalyze the oxidative deamination of amines, such as dopamine, norepinephrine, and serotonin. A mutation of this gene results in Brunner syndrome. This gene has also been associated with a variety of other psychiatric disorders, including antisocial behavior. Alternatively spliced transcript variants encoding multiple isoforms have been observed.[7]
| MAOA | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | MAOA, MAO-A, monoamine oxidase A, BRNRS | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 309850 MGI: 96915 HomoloGene: 203 GeneCards: MAOA | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Structures
Gene
Monoamine oxidase A, also known as MAO-A, is an enzyme that in humans is encoded by the MAOA gene.[5][6] The promoter of MAOA contains conserved binding sites for Sp1, GATA2, and TBP.[8] This gene is adjacent to a related gene (MAOB) on the opposite strand of the X chromosome.[9]
In humans, there is a 30-base repeat sequence repeated several different numbers of times in the promoter region of MAO-A. There are 2R (two repeats), 3R, 3.5R, 4R, and 5R variants of the repeat sequence, with the 3R and 4R variants most common in all populations. The variants of the promoter have been found to appear at different frequencies in different ethnic groups in an American sample cohort.[10]
The epigenetic modification of MAOA gene expression through methylation likely plays an important role in women.[11] A study from 2010 found epigenetic methylation of MAOA in men to be very low and with little variability compared to women, while having higher heritability in men than women.[12]
Protein
MAO-A shares 70% amino acid sequence identity with its homologue MAO-B.[13] Accordingly, both proteins have similar structures. Both MAO-A and MAO-B exhibit an N-terminal domain that binds flavin adenine dinucleotide (FAD), a central domain that binds the amine substrate, and a C-terminal α-helix that is inserted in the outer mitochondrial membrane.[13][14] MAO-A has a slightly larger substrate-binding cavity than MAO-B, which may be the cause of slight differences in catalytic activity between the two enzymes, as shown in quantitative structure-activity relationship experiments.[15] Both enzymes are relatively large, about 60 kilodaltons in size, and are believed to function as dimers in living cells.[14]
Function
Monoamine oxidase A catalyzes O2-dependent oxidation of primary arylalkyl amines, most importantly neurotransmitters such as dopamine and serotonin. This is the initial step in the breakdown of these molecules. The products are the corresponding aldehyde, hydrogen peroxide, and ammonia:
- RCH
2-Amine + O
2 + H
2O → R-Aldehyde + H
2O
2 + NH
3
This reaction is believed to occur in three steps, using FAD as an electron-transferring cofactor. First, the amine is oxidized to the corresponding imine, with reduction of FAD to FADH2. Second, O2 accepts two electrons and two protons from FADH2, forming H
2O
2 and regenerating FAD. Third, the imine is hydrolyzed by water, forming ammonia and the aldehyde.[15][16]
Compared to MAO-B, MAO-A has a higher specificity for serotonin and norepinephrine, while the two enzymes have similar affinity for dopamine and tyramine.[17]
MAO-A is a key regulator for normal brain function. In the brain, the highest levels of transcription occur in the brain stem, hypothalamus, amygdala, habenula, and nucleus accumbens, and the lowest in the thalamus, spinal cord, pituitary gland, and cerebellum.[17] Its expression is regulated by the transcription factors SP1, GATA2, and TBP via cAMP-dependent regulation.[8][17] MAO-A is also expressed in cardiomyocytes, where it is induced in response to stress such as ischemia and inflammation.[8]
Clinical significance
Cancer
MAO-A produces an amine oxidase, which is a class of enzyme known to affect carcinogenesis. Clorgyline, an MAO-A enzyme inhibitor, prevents apoptosis in melanoma cells, in vitro.[18] Cholangiocarcinoma suppresses MAO-A expression, and those patients with higher MAO-A expression had less adjacent organ invasion and better prognosis and survival.[19]
Cardiovascular disease
MAOA activity is linked to apoptosis and cardiac damage during cardiac injury following ischemic-reperfusion.[8]
Behavioral and neurological disorders
There is some association between low activity forms of the MAOA gene and autism.[20] Mutations in the MAOA gene results in monoamine oxidase deficiency, or Brunner syndrome.[7] Other disorders associated with MAO-A include Alzheimer's disease, aggression, panic disorder, bipolar disorder, major depressive disorder, and attention deficit hyperactivity disorder.[8] Effects of parenting on self-regulation in adolescents appear to be moderated by 'plasticity alleles', of which the 2R and 3R alleles of MAOA are two, with "the more plasticity alleles males (but not females) carried, the more and less self-regulation they manifested under, respectively, supportive and unsupportive parenting conditions."[21]
Depression
MAO-A levels in the brain as measured using positron emission tomography are elevated by an average of 34% in patients with major depressive disorder.[22] Genetic association studies examining the relationship between high-activity MAOA variants and depression have produced mixed results, with some studies linking the high-activity variants to major depression in females,[23] depressed suicide in males,[24] major depression and sleep disturbance in males[25] and major depressive disorder in both males and females.[26]
Other studies failed to find a significant relationship between high-activity variants of the MAOA gene and major depressive disorder.[27][28] In patients with major depressive disorder, those with MAOA G/T polymorphisms (rs6323) coding for the highest-activity form of the enzyme have a significantly lower magnitude of placebo response than those with other genotypes.[29]
Antisocial behavior
In humans, an association between the 2R allele of the VNTR region of the gene and an increase in the likelihood of committing serious crime or violence has been found. The VNTR 2R allele of MAOA has been found to be a risk factor for violent delinquency, when present in association with stresses, i.e. family issues, low popularity or failing school.[30][31][32][33]
A connection between the MAO-A gene 3R version and several types of anti-social behaviour has been found: Maltreated children with genes causing high levels of MAO-A were less likely to develop antisocial behavior.[34] Low MAO-A activity alleles which are overwhelmingly the 3R allele in combination with abuse experienced during childhood resulted in an increased risk of aggressive behaviour as an adult,[35] and men with the low activity MAOA allele were more genetically vulnerable even to punitive discipline as a predictor of antisocial behaviour.[36] High testosterone, maternal tobacco smoking during pregnancy, poor material living standards, dropping out of school, and low IQ predicted violent behavior are associated with men with the low-activity alleles.[37][38] According to a large meta-analysis in 2014, the 3R allele had a small, nonsignificant effect on aggression and antisocial behavior, in the absence of other interaction factors. Owing to methodological concerns, the authors do not view this as evidence in favor of an effect.[39]
The MAO-A gene was the first candidate gene for antisocial behavior and was identified during a "molecular genetic analysis of a large, multigenerational, and notoriously violent, Dutch kindred".[40] A study of Finnish prisoners revealed that a MAOA-L (low-activity) genotype, which contributes to low dopamine turnover rate, was associated with extremely violent behavior.[41] For the purpose of the study, "extremely violent behavior" was defined as at least ten committed homicides, attempted homicides or batteries.
However, a large genome-wide association study has failed to find any large or statistically significant effects of the MAOA gene on aggression.[42] A separate GWAS on antisocial personality disorder likewise did not report a significant effect of MAOA.[43] Another study, while finding effects from a candidate gene search, failed to find any evidence in a large GWAS.[41] A separate analysis of human and rat genome wide association studies, Mandelian randomization studies, and causal pathway analyses likewise failed to reveal robust evidence of MAOA in aggression.[44] This lack of replication is predicted from the known issues of candidate gene research, which can produce many substantial false positives.[45]
Aggression and the "Warrior gene"
Low-activity variants of the VNTR promoter region of the MAO-A gene have been referred to as the warrior gene.[46] When faced with social exclusion or ostracism, individuals with the low activity MAO-A variants showed higher levels of aggression than individuals with the high activity MAO-A gene.[47] Low activity MAO-A could significantly predict aggressive behaviour in a high provocation situation: Individuals with the low activity variant of the MAO-A gene were more likely (75% as opposed to 62%, out of a sample size of 70) to retaliate, and with greater force, as compared to those with a normal MAO-A variant if the perceived loss was large.[48]
The effects of MAOA genes on aggression have also been criticized for being heavily overstated.[49] Indeed, the MAOA gene, even in conjunction with childhood adversity, is known to have a very small effect.[50] The vast majority of people with the associated alleles have not committed any violent acts.[51][52]
Legal implications
In a 2009 criminal trial in the United States, an argument based on a combination of "warrior gene" and history of child abuse was successfully used to avoid a conviction of first-degree murder and the death penalty; however, the convicted murderer was sentenced to 32 years in prison.[53][54] In a second case, an individual was convicted of second-degree murder, rather than first-degree murder, based on a genetic test that revealed he had the low-activity MAOA variant.[55] Judges in Germany are more likely to sentence offenders to involuntary psychiatric hospitalization on hearing an accused's MAOA-L genotype.[56]
Epigenetics
Studies have linked methylation of the MAOA gene with nicotine and alcohol dependence in women.[57] A second MAOA VNTR promoter, P2, influences epigenetic methylation and interacts with having experienced child abuse to influence antisocial personality disorder symptoms, only in women.[58] A study of 34 non-smoking men found that methylation of the gene may alter its expression in the brain.[59]
Animal studies
A dysfunctional MAOA gene has been correlated with increased aggression levels in mice,[60][61] and has been correlated with heightened levels of aggression in humans.[62] In mice, a dysfunctional MAOA gene is created through insertional mutagenesis (called 'Tg8').[60] Tg8 is a transgenic mouse strain that lacks functional MAO-A enzymatic activity. Mice that lacked a functional MAOA gene exhibited increased aggression towards intruder mice.[60][63]
Some types of aggression exhibited by these mice were territorial aggression, predatory aggression, and isolation-induced aggression.[61] The MAO-A deficient mice that exhibited increased isolation-induced aggression reveals that an MAO-A deficiency may also contribute to a disruption in social interactions.[64] There is research in both humans and mice to support that a nonsense point mutation in the eighth exon of the MAOA gene is responsible for impulsive aggressiveness due to a complete MAO-A deficiency.[60][62]
Interactions
Transcription factors
A number of transcription factors bind to the promoter region of MAO-A and upregulate its expression. These include:Sp1 transcription factor, GATA2, TBP.[8]
Inducers
Synthetic compounds that up-regulate the expression of MAO-A include Valproic acid (Depakote)[65]
Inhibitors
Substances that inhibit the enzymatic activity of MAO-A include:
- Synthetic compounds
- Befloxatone (MD370503)
- Brofaromine (Consonar)
- Cimoxatone
- Clorgyline (irreversible)
- Methylene Blue
- Minaprine (Cantor)
- Moclobemide (Aurorix, Manerix)
- Phenelzine (Nardil)
- Pirlindole (Pirazidol)
- Toloxatone (Humoryl)
- Tyrima (CX 157)
- Tranylcypromine (nonselective and irreversible)
- Natural products
- Incarviatone A
- Herbal sources
See also
- Monoamine oxidase B
- Monoamine oxidase inhibitor - a class of antidepressant drugs that block or inactivate one or both MAO isoforms
References
- GRCh38: Ensembl release 89: ENSG00000189221 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000025037 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Hotamisligil GS, Breakefield XO (August 1991). "Human monoamine oxidase A gene determines levels of enzyme activity". American Journal of Human Genetics. 49 (2): 383–92. PMC 1683299. PMID 1678250.
- Grimsby J, Chen K, Wang LJ, Lan NC, Shih JC (May 1991). "Human monoamine oxidase A and B genes exhibit identical exon-intron organization". Proceedings of the National Academy of Sciences of the United States of America. 88 (9): 3637–41. Bibcode:1991PNAS...88.3637G. doi:10.1073/pnas.88.9.3637. PMC 51507. PMID 2023912.
- "Entrez Gene: MAOA monoamine oxidase A".
- Gupta V, Khan AA, Sasi BK, Mahapatra NR (July 2015). "Molecular mechanism of monoamine oxidase A gene regulation under inflammation and ischemia-like conditions: key roles of the transcription factors GATA2, Sp1 and TBP". Journal of Neurochemistry. 134 (1): 21–38. doi:10.1111/jnc.13099. PMID 25810277. S2CID 21044944.
- Eccles DA, Macartney-Coxson D, Chambers GK, Lea RA (10 January 2012). "A unique demographic history exists for the MAO-A gene in Polynesians". Journal of Human Genetics. 57 (5): 294–300. doi:10.1038/jhg.2012.19. PMID 22377710.
- Sabol SZ, Hu S, Hamer D (September 1998). "A functional polymorphism in the monoamine oxidase A gene promoter". Human Genetics. 103 (3): 273–9. doi:10.1007/s004390050816. PMID 9799080. S2CID 29954052.
- Jiang Y, Langley B, Lubin FD, Renthal W, Wood MA, Yasui DH, et al. (November 2008). "Epigenetics in the nervous system". The Journal of Neuroscience. 28 (46): 11753–9. doi:10.1523/JNEUROSCI.3797-08.2008. PMC 3844836. PMID 19005036.
- Wong CC, Caspi A, Williams B, Craig IW, Houts R, Ambler A, et al. (August 2010). "A longitudinal study of epigenetic variation in twins". Epigenetics. 5 (6): 516–26. doi:10.4161/epi.5.6.12226. PMC 3322496. PMID 20505345.
- Binda C, Mattevi A, Edmondson DE (2011). "Structural properties of human monoamine oxidases a and B". Monoamine Oxidase and their Inhibitors. International Review of Neurobiology. Vol. 100. pp. 1–11. doi:10.1016/B978-0-12-386467-3.00001-7. ISBN 9780123864673. PMID 21971000.
- Iacovino LG, Magnani F, Binda C (November 2018). "The structure of monoamine oxidases: past, present, and future". Journal of Neural Transmission. 125 (11): 1567–1579. doi:10.1007/s00702-018-1915-z. PMID 30167931.
- Edmondson DE, Binda C, Mattevi A (August 2007). "Structural insights into the mechanism of amine oxidation by monoamine oxidases A and B". Archives of Biochemistry and Biophysics. 464 (2): 269–276. doi:10.1016/j.abb.2007.05.006. PMC 1993809. PMID 17573034.
- Binda C, Mattevi A, Edmondson DE (July 5, 2002). "Structure-function relationships in flavoenzyme-dependent amine oxidations: A comparison of polyamine oxidase and monoamine oxidase". Journal of Biological Chemistry. 277 (27): 23973–23976. doi:10.1074/jbc.R200005200.
- Kolla NJ, Bortolato M (June 2020). "The role of monoamine oxidase A in the neurobiology of aggressive, antisocial, and violent behavior: A tale of mice and men". Progress in Neurobiology. 194: 101875. doi:10.1016/j.pneurobio.2020.101875. PMC 7609507.
- Pietrangeli P, Mondovì B (January 2004). "Amine oxidases and tumors". Neurotoxicology. 25 (1–2): 317–24. doi:10.1016/S0161-813X(03)00109-8. PMID 14697906.
- Huang L, Frampton G, Rao A, Zhang KS, Chen W, Lai JM, et al. (October 2012). "Monoamine oxidase A expression is suppressed in human cholangiocarcinoma via coordinated epigenetic and IL-6-driven events". Laboratory Investigation; A Journal of Technical Methods and Pathology. 92 (10): 1451–60. doi:10.1038/labinvest.2012.110. PMC 3959781. PMID 22906985.
- Cohen IL, Liu X, Lewis ME, Chudley A, Forster-Gibson C, Gonzalez M, et al. (April 2011). "Autism severity is associated with child and maternal MAOA genotypes". Clinical Genetics. 79 (4): 355–62. doi:10.1111/j.1399-0004.2010.01471.x. PMID 20573161. S2CID 24366751.
- Belsky J, Beaver KM (May 2011). "Cumulative-genetic plasticity, parenting and adolescent self-regulation". Journal of Child Psychology and Psychiatry, and Allied Disciplines. 52 (5): 619–26. doi:10.1111/j.1469-7610.2010.02327.x. PMC 4357655. PMID 21039487.
- Meyer JH, Ginovart N, Boovariwala A, Sagrati S, Hussey D, Garcia A, et al. (November 2006). "Elevated monoamine oxidase a levels in the brain: an explanation for the monoamine imbalance of major depression". Archives of General Psychiatry. 63 (11): 1209–16. doi:10.1001/archpsyc.63.11.1209. PMID 17088501.
- Schulze TG, Müller DJ, Krauss H, Scherk H, Ohlraun S, Syagailo YV, et al. (December 2000). "Association between a functional polymorphism in the monoamine oxidase A gene promoter and major depressive disorder". American Journal of Medical Genetics. 96 (6): 801–3. doi:10.1002/1096-8628(20001204)96:6<801::AID-AJMG21>3.0.CO;2-4. PMID 11121185.
- Du L, Faludi G, Palkovits M, Sotonyi P, Bakish D, Hrdina PD (July 2002). "High activity-related allele of MAO-A gene associated with depressed suicide in males". NeuroReport. 13 (9): 1195–8. doi:10.1097/00001756-200207020-00025. PMID 12151768. S2CID 19874514.
- Du L, Bakish D, Ravindran A, Hrdina PD (September 2004). "MAO-A gene polymorphisms are associated with major depression and sleep disturbance in males". NeuroReport. 15 (13): 2097–101. doi:10.1097/00001756-200409150-00020. PMID 15486489. S2CID 39844598.
- Yu YW, Tsai SJ, Hong CJ, Chen TJ, Chen MC, Yang CW (September 2005). "Association study of a monoamine oxidase a gene promoter polymorphism with major depressive disorder and antidepressant response". Neuropsychopharmacology. 30 (9): 1719–23. doi:10.1038/sj.npp.1300785. PMID 15956990.
- Serretti A, Cristina S, Lilli R, Cusin C, Lattuada E, Lorenzi C, et al. (May 2002). "Family-based association study of 5-HTTLPR, TPH, MAO-A, and DRD4 polymorphisms in mood disorders". American Journal of Medical Genetics. 114 (4): 361–9. doi:10.1002/ajmg.10356. PMID 11992558.
- Huang SY, Lin MT, Lin WW, Huang CC, Shy MJ, Lu RB (2009). "Association of monoamine oxidase A (MAOA) polymorphisms and clinical subgroups of major depressive disorders in the Han Chinese population". The World Journal of Biological Psychiatry. 10 (4 Pt 2): 544–51. doi:10.1080/15622970701816506. PMID 19224413. S2CID 30281258.
- Leuchter AF, McCracken JT, Hunter AM, Cook IA, Alpert JE (August 2009). "Monoamine oxidase a and catechol-o-methyltransferase functional polymorphisms and the placebo response in major depressive disorder". Journal of Clinical Psychopharmacology. 29 (4): 372–7. doi:10.1097/JCP.0b013e3181ac4aaf. PMID 19593178. S2CID 29200403.
- Garcia-Arocena D. "The Genetics of Violent Behavior". The Jackson Laboratory. The Jackson Laboratory. Retrieved 2021-03-23.
- Guo G, Ou XM, Roettger M, Shih JC (May 2008). "The VNTR 2 repeat in MAOA and delinquent behavior in adolescence and young adulthood: associations and MAOA promoter activity". European Journal of Human Genetics. 16 (5): 626–34. doi:10.1038/sj.ejhg.5201999. PMC 2922855. PMID 18212819.
- Guo G, Roettger M, Shih JC (August 2008). "The integration of genetic propensities into social-control models of delinquency and violence among male youths". American Sociological Review. 73 (4): 543–568. doi:10.1177/000312240807300402. S2CID 30271933.
- Beaver KM, Wright JP, Boutwell BB, Barnes JC, DeLisi M, Vaughn MG (2012). "Exploring the association between the 2-repeat allele of the MAOA gene promoter polymorphism and psychopathic personality traits, arrests, incarceration, and lifetime antisocial behavior". Personality and Individual Differences. 54 (2): 164–168. doi:10.1016/j.paid.2012.08.014.
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, et al. (August 2002). "Role of genotype in the cycle of violence in maltreated children". Science. 297 (5582): 851–4. Bibcode:2002Sci...297..851C. doi:10.1126/science.1072290. PMID 12161658. S2CID 7882492.
- "Gene may protect abused kids against behavior problems". EurekAlert! (Press release). 2002-08-01.
- Frazzetto G, Di Lorenzo G, Carola V, Proietti L, Sokolowska E, Siracusano A, et al. (May 2007). "Early trauma and increased risk for physical aggression during adulthood: the moderating role of MAOA genotype". PLOS ONE. 2 (5): e486. Bibcode:2007PLoSO...2..486F. doi:10.1371/journal.pone.0000486. PMC 1872046. PMID 17534436.
- Choe DE, Shaw DS, Hyde LW, Forbes EE (September 2014). "Interactions Between Monoamine Oxidase A and Punitive Discipline in African American and Caucasian Men's Antisocial Behavior". Clinical Psychological Science. 2 (5): 591–601. doi:10.1177/2167702613518046. PMC 4802365. PMID 27014508.
- Fergusson DM, Boden JM, Horwood LJ, Miller A, Kennedy MA (February 2012). "Moderating role of the MAOA genotype in antisocial behaviour". The British Journal of Psychiatry. 200 (2): 116–23. doi:10.1192/bjp.bp.111.093328. PMC 3269651. PMID 22297589.
- Sjöberg RL, Ducci F, Barr CS, Newman TK, Dell'osso L, Virkkunen M, Goldman D (January 2008). "A non-additive interaction of a functional MAO-A VNTR and testosterone predicts antisocial behavior". Neuropsychopharmacology. 33 (2): 425–30. doi:10.1038/sj.npp.1301417. PMC 2665792. PMID 17429405.
- Ficks CA, Waldman ID (September 2014). "Candidate genes for aggression and antisocial behavior: a meta-analysis of association studies of the 5HTTLPR and MAOA-uVNTR". Behavior Genetics. 44 (5): 427–44. doi:10.1007/s10519-014-9661-y. PMID 24902785. S2CID 11599122.
- Dorfman HM, Meyer-Lindenberg A, Buckholtz JW (2014). "Neurobiological mechanisms for impulsive-aggression: the role of MAOA". Current Topics in Behavioral Neurosciences. 17: 297–313. doi:10.1007/7854_2013_272. ISBN 978-3-662-44280-7. PMID 24470068.
- Tiihonen J, Rautiainen MR, Ollila HM, Repo-Tiihonen E, Virkkunen M, Palotie A, Pietiläinen O, Kristiansson K, Joukamaa M, Lauerma H, Saarela J, Tyni S, Vartiainen H, Paananen J, Goldman D, Paunio T (June 2015). "Genetic Background of Extreme Violent Behavior". Molecular Psychiatry. 20 (6): 786–92. doi:10.1038/mp.2014.130. PMC 4776744. PMID 25349169.
- Vassos E, Collier DA, Fazel S (April 2014). "Systematic meta-analyses and field synopsis of genetic association studies of violence and aggression". Molecular Psychiatry. 19 (4): 471–7. doi:10.1038/mp.2013.31. PMC 3965568. PMID 23546171. S2CID 13936647.
- Rautiainen MR, Paunio T, Repo-Tiihonen E, Virkkunen M, Ollila HM, Sulkava S, et al. (September 2016). "Genome-wide association study of antisocial personality disorder". Translational Psychiatry. 6 (9): e883. doi:10.1038/tp.2016.155. PMC 5048197. PMID 27598967.
- Zhang-James Y, Fernàndez-Castillo N, Hess JL, Malki K, Glatt SJ, Cormand B, Faraone SV (November 2019). "An integrated analysis of genes and functional pathways for aggression in human and rodent models". Molecular Psychiatry. 24 (11): 1655–1667. doi:10.1038/s41380-018-0068-7. PMC 6274606. PMID 29858598.
- Sullivan PF (May 2007). "Spurious genetic associations". Biological Psychiatry. 61 (10): 1121–6. doi:10.1016/j.biopsych.2006.11.010. PMID 17346679. S2CID 35033987.
- Hogenboom M (28 October 2014). "Two genes linked with violent crime". BBC News. Retrieved 2014-11-01.
- Gallardo-Pujol D, Andrés-Pueyo A, Maydeu-Olivares A (February 2013). "MAOA genotype, social exclusion and aggression: an experimental test of a gene-environment interaction". Genes, Brain and Behavior. 12 (1): 140–5. doi:10.1111/j.1601-183X.2012.00868.x. PMID 23067570. S2CID 4830611.
- McDermott R, Tingley D, Cowden J, Frazzetto G, Johnson DD (February 2009). "Monoamine oxidase A gene (MAOA) predicts behavioral aggression following provocation". Proceedings of the National Academy of Sciences of the United States of America. 106 (7): 2118–23. Bibcode:2009PNAS..106.2118M. doi:10.1073/pnas.0808376106. PMC 2650118. PMID 19168625.
- Horgan J (26 April 2011). "Code rage: The "warrior gene" makes me mad! (Whether I have it or not)". Scientific American.
- Hovet K (20 February 2018). "Chasing the 'warrior gene' and why it looks like a dud so far". Genetic Literacy Project.
- Powledge TM (29 July 2016). "Do the MAOA and CDH13 'human warrior genes' make violent criminals—and what should society do?". Genetic Literacy Project.
- "MAOA and CDH13 genes linked to violent crime, but can they explain criminal behavior?". Genetic Literacy Project. 29 October 2014.
- Barber N (2010-07-13). "Pity the poor murderer, his genes made him do it". Psychology Today. Blog: "The Human Beast: Why we do what we do". Retrieved 2010-10-17.
- Hagerty BB (2010-07-01). "Can Your Genes Make You Murder?". NPR.org. National Public Radio. Retrieved 2010-10-17.
- Scurich N, Appelbaum PS (July 2021). "State v. Yepez: Admissibility and Relevance of Behavioral Genetic Evidence in a Criminal Trial". Psychiatric Services. 72 (7): 853–855. doi:10.1176/appi.ps.202100226. PMID 34074149. S2CID 235298342.
- McSwiggan S, Elger B, Appelbaum PS (January 1, 2017). "The forensic use of behavioral genetics in criminal proceedings: Case of the MAOA-L genotype". International Journal of Law and Psychiatry. 50: 17–23. doi:10.1016/j.ijlp.2016.09.005. PMC 5250535. PMID 27823806.
- Philibert RA, Gunter TD, Beach SR, Brody GH, Madan A (July 2008). "MAOA methylation is associated with nicotine and alcohol dependence in women". American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 147B (5): 565–70. doi:10.1002/ajmg.b.30778. PMC 3685146. PMID 18454435.
- Philibert RA, Wernett P, Plume J, Packer H, Brody GH, Beach SR (July 2011). "Gene environment interactions with a novel variable Monoamine Oxidase A transcriptional enhancer are associated with antisocial personality disorder". Biological Psychology. 87 (3): 366–71. doi:10.1016/j.biopsycho.2011.04.007. PMC 3134149. PMID 21554924.
- Shumay E, Logan J, Volkow ND, Fowler JS (October 2012). "Evidence that the methylation state of the monoamine oxidase A (MAOA) gene predicts brain activity of MAO A enzyme in healthy men". Epigenetics. 7 (10): 1151–1160. doi:10.4161/epi.21976. PMC 3469457. PMID 22948232.
- Scott AL, Bortolato M, Chen K, Shih JC (May 2008). "Novel monoamine oxidase A knock out mice with human-like spontaneous mutation". NeuroReport. 19 (7): 739–43. doi:10.1097/WNR.0b013e3282fd6e88. PMC 3435113. PMID 18418249.
- Vishnivetskaya GB, Skrinskaya JA, Seif I, Popova NK (2007). "Effect of MAO A deficiency on different kinds of aggression and social investigation in mice". Aggressive Behavior. 33 (1): 1–6. doi:10.1002/ab.20161. PMID 17441000.
- Brunner HG, Nelen M, Breakefield XO, Ropers HH, van Oost BA (October 1993). "Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A". Science. 262 (5133): 578–80. Bibcode:1993Sci...262..578B. doi:10.1126/science.8211186. PMID 8211186.
- Vishnivetskaya GB, Skrinskaya JA, Seif I, Popova NK (1 January 2007). "Effect of MAO A deficiency on different kinds of aggression and social investigation in mice". Aggressive Behavior. 33 (1): 1–6. doi:10.1002/ab.20161. PMID 17441000.
- Hebebrand J, Klug B (September 1995). "Specification of the phenotype required for men with monoamine oxidase type A deficiency". Human Genetics. 96 (3): 372–6. doi:10.1007/BF00210430. PMID 7649563. S2CID 33294633.
- Wu JB, Shih JC (October 2011). "Valproic acid induces monoamine oxidase A via Akt/forkhead box O1 activation". Molecular Pharmacology. 80 (4): 714–23. doi:10.1124/mol.111.072744. PMC 3187529. PMID 21775495.
- Lee SA, Hong SS, Han XH, Hwang JS, Oh GJ, Lee KS, et al. (July 2005). "Piperine from the fruits of Piper longum with inhibitory effect on monoamine oxidase and antidepressant-like activity". Chemical & Pharmaceutical Bulletin. 53 (7): 832–5. doi:10.1248/cpb.53.832. PMID 15997146.
- van Diermen D, Marston A, Bravo J, Reist M, Carrupt PA, Hostettmann K (March 2009). "Monoamine oxidase inhibition by Rhodiola rosea L. roots". Journal of Ethnopharmacology. 122 (2): 397–401. doi:10.1016/j.jep.2009.01.007. PMID 19168123.
Further reading
- Rehan W, Sandnabba NK, Johansson A, Westberg L, Santtila P (October 2015). "Effects of MAOA genotype and childhood experiences of physical and emotional abuse on aggressive behavior in adulthood". Nordic Psychology. 67 (4): 301–12. doi:10.1080/19012276.2015.1026922. S2CID 146577097.
- McDermott R, Tingley D, Cowden J, Frazzetto G, Johnson DD (February 2009). "Monoamine oxidase A gene (MAOA) predicts behavioral aggression following provocation". Proceedings of the National Academy of Sciences of the United States of America. 106 (7): 2118–23. Bibcode:2009PNAS..106.2118M. doi:10.1073/pnas.0808376106. PMC 2650118. PMID 19168625.
- Edmondson DE, Binda C, Mattevi A (January 2004). "The FAD binding sites of human monoamine oxidases A and B". Neurotoxicology. 25 (1–2): 63–72. doi:10.1016/S0161-813X(03)00114-1. PMID 14697881.
- Craig IW (March 2007). "The importance of stress and genetic variation in human aggression". BioEssays. 29 (3): 227–36. doi:10.1002/bies.20538. PMID 17295220. S2CID 46059787.
External links
- Overview of all the structural information available in the PDB for UniProt: P21397 (Human Monoamine oxidase A) at the PDBe-KB.