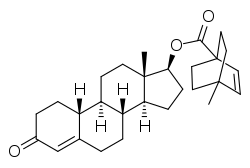
Nandrolone cyclotate
Nandrolone cyclotate (USANTooltip United States Adopted Name) (developmental code name RS-3268R), or nandrolone ciclotate, also known as 19-nortestosterone 17β-ciclotate, is a synthetic and injected anabolic–androgenic steroid (AAS) of the nandrolone (19-nortestosterone) group which was never marketed.[1][2][3][4] It is an androgen ester – specifically, the C17β ciclotate (4-methylbicyclo[2.2.2]oct-2-ene-1-carboxylate) ester of nandrolone.[1][2] Nandrolone cyclotate has potent and prolonged activity as an AAS when administered by intramuscular injection and is reported to have a similar duration of action to that of nandrolone decanoate via this route.[4]
| Compound | PRTooltip Progesterone receptor | ARTooltip Androgen receptor | ERTooltip Estrogen receptor | GRTooltip Glucocorticoid receptor | MRTooltip Mineralocorticoid receptor | SHBGTooltip Sex hormone-binding globulin | CBGTooltip Corticosteroid-binding globulin |
|---|---|---|---|---|---|---|---|
| Nandrolone | 20 | 154–155 | <0.1 | 0.5 | 1.6 | 1–16 | 0.1 |
| Testosterone | 1.0–1.2 | 100 | <0.1 | 0.17 | 0.9 | 19–82 | 3–8 |
| Estradiol | 2.6 | 7.9 | 100 | 0.6 | 0.13 | 8.7–12 | <0.1 |
| Notes: Values are percentages (%). Reference ligands (100%) were progesterone for the PRTooltip progesterone receptor, testosterone for the ARTooltip androgen receptor, estradiol for the ERTooltip estrogen receptor, dexamethasone for the GRTooltip glucocorticoid receptor, aldosterone for the MRTooltip mineralocorticoid receptor, dihydrotestosterone for SHBGTooltip sex hormone-binding globulin, and cortisol for CBGTooltip corticosteroid-binding globulin. Sources: See template. | |||||||
 | |
| Clinical data | |
|---|---|
| Other names | Nandrolone ciclotate; RS-3268R; 19-Nortestosterone 17β-ciclotate |
| Routes of administration | Intramuscular injection |
| Drug class | Androgen; Anabolic steroid; Androgen ester; Progestogen |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C28H38O3 |
| Molar mass | 422.609 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
References
- Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 660–. ISBN 978-1-4757-2085-3.
- Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 716–717. ISBN 978-3-88763-075-1.
- Morton IK, Hall JM (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. ISBN 978-94-011-4439-1.
- van Wayjen RG, van den Ende A (May 1973). "Clinical-pharmacological investigation of the nitrogen-retaining effect of nandrolone cyclotate". Acta Endocrinologica. 73 (1): 171–8. doi:10.1530/acta.0.0730171. PMID 4740146.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.