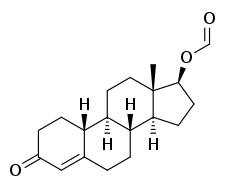
Nandrolone formate
Nandrolone formate, also known as nandrolone carboxylate or nandrolone methanoate, as well as 19-nortestosterone 17β-formate or estr-4-en-17β-ol-3-one 17β-formate, is a synthetic, injected anabolic–androgenic steroid (AAS) and a derivative of 19-nortestosterone (nandrolone) that was never marketed.[1][2] It is an androgen ester – specifically, the C17β formate ester of nandrolone.[1][2]
| Compound | PRTooltip Progesterone receptor | ARTooltip Androgen receptor | ERTooltip Estrogen receptor | GRTooltip Glucocorticoid receptor | MRTooltip Mineralocorticoid receptor | SHBGTooltip Sex hormone-binding globulin | CBGTooltip Corticosteroid-binding globulin |
|---|---|---|---|---|---|---|---|
| Nandrolone | 20 | 154–155 | <0.1 | 0.5 | 1.6 | 1–16 | 0.1 |
| Testosterone | 1.0–1.2 | 100 | <0.1 | 0.17 | 0.9 | 19–82 | 3–8 |
| Estradiol | 2.6 | 7.9 | 100 | 0.6 | 0.13 | 8.7–12 | <0.1 |
| Notes: Values are percentages (%). Reference ligands (100%) were progesterone for the PRTooltip progesterone receptor, testosterone for the ARTooltip androgen receptor, estradiol for the ERTooltip estrogen receptor, dexamethasone for the GRTooltip glucocorticoid receptor, aldosterone for the MRTooltip mineralocorticoid receptor, dihydrotestosterone for SHBGTooltip sex hormone-binding globulin, and cortisol for CBGTooltip corticosteroid-binding globulin. Sources: See template. | |||||||
 | |
| Clinical data | |
|---|---|
| Other names | Nandrolone carboxylate; Nandrolone methanoate; 19-Nortestosterone 17β-formate; Estr-4-en-17β-ol-3-one 17β-formate |
| Routes of administration | Intramuscular injection |
| Drug class | Androgen; Anabolic steroid; Androgen ester; Progestogen |
| Identifiers | |
| |
| CAS Number | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C19H26O3 |
| Molar mass | 302.414 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
References
- Chaudry MA, James KC, Ng CT, Nicholls PJ (1976). "Anabolic and androgenic activities, in rat, of some nandrolone and androstanolone esters". J. Pharm. Pharmacol. 28 (12): 882–5. doi:10.1111/j.2042-7158.1976.tb04085.x. PMID 12263. S2CID 20546783.
- Abolghasem Jouyban (26 August 2009). Handbook of Solubility Data for Pharmaceuticals. CRC Press. pp. 125–. ISBN 978-1-4398-0488-9.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.