Dinitrogen pentoxide
Dinitrogen pentoxide (also known as nitrogen pentoxide or nitric anhydride) is the chemical compound with the formula N2O5. It is one of the binary nitrogen oxides, a family of compounds that only contain nitrogen and oxygen. It exists as colourless crystals that sublime slightly above room temperature, yielding a colorless gas.[4]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Dinitrogen pentoxide | |
| Other names
Nitric anhydride Nitronium nitrate Nitryl nitrate DNPO Anhydrous nitric acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.030.227 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| N2O5 | |
| Molar mass | 108.01 g/mol |
| Appearance | white solid |
| Density | 2.0 g/cm3[1] |
| Boiling point | 33 °C (91 °F; 306 K) sublimes[1] |
| reacts to give HNO3 | |
| Solubility | soluble in chloroform negligible in CCl4 |
| −35.6×10−6 cm3 mol−1 (aq) | |
| 1.39 D | |
| Structure[2] | |
| Hexagonal, hP14 | |
| P63/mmc No. 194 | |
a = 0.54019 nm, c = 0.65268 nm | |
Formula units (Z) |
2 |
| planar, C2v (approx. D2h) N–O–N ≈ 180° | |
| Thermochemistry[3] | |
Heat capacity (C) |
143.1 J K−1 mol−1 (s) 95.3 J K−1 mol−1 (g) |
Std molar entropy (S⦵298) |
178.2 J K−1 mol−1 (s) 355.7 J K−1 mol−1 (g) |
Std enthalpy of formation (ΔfH⦵298) |
−43.1 kJ/mol (s) +13.3 kJ/mol (g) |
Gibbs free energy (ΔfG⦵) |
113.9 kJ/mol (s) +117.1 kJ/mol (g) |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
strong oxidizer, forms strong acid in contact with water |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Related compounds | |
| Nitrous oxide Nitric oxide Dinitrogen trioxide Nitrogen dioxide Dinitrogen tetroxide | |
Related compounds |
Nitric acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Dinitrogen pentoxide is an unstable and potentially dangerous oxidizer that once was used as a reagent when dissolved in chloroform for nitrations but has largely been superseded by nitronium tetrafluoroborate (NO2BF4).
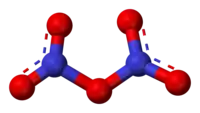
N2O5 is a rare example of a compound that adopts two structures depending on the conditions. The solid is a salt, nitronium nitrate, consisting of separate nitronium cations [NO2]+ and nitrate anions [NO3]−; but in the gas phase and under some other conditions it is a covalently-bound molecule.[5]
History
N2O5 was first reported by Deville in 1840, who prepared it by treating silver nitrate (AgNO3) with chlorine.[6][7]
Structure and physical properties
Pure solid N2O5 is a salt, consisting of separated linear nitronium ions NO+2 and planar trigonal nitrate anions NO−3. Both nitrogen centers have oxidation state +5. It crystallizes in the space group D4
6h (C6/mmc) with Z = 2, with the NO−3 anions in the D3h sites and the NO+2 cations in D3d sites.[8]
The vapor pressure P (in atm) as a function of temperature T (in kelvin), in the range 211 to 305 K (−62 to 32 °C), is well approximated by the formula
being about 48 torr at 0 °C, 424 torr at 25 °C, and 760 torr at 32 °C (9 °C below the melting point).[9]
In the gas phase, or when dissolved in nonpolar solvents such as carbon tetrachloride, the compound exists as covalently-bonded molecules O2N−O−NO2. In the gas phase, theoretical calculations for the minimum-energy configuration indicate that the O−N−O angle in each −NO2 wing is about 134° and the N−O−N angle is about 112°. In that configuration, the two −NO2 groups are rotated about 35° around the bonds to the central oxygen, away from the N−O−N plane. The molecule thus has a propeller shape, with one axis of 180° rotational symmetry (C2) [10]
When gaseous N2O5 is cooled rapidly ("quenched"), one can obtain the metastable molecular form, which exothermically converts to the ionic form above −70 °C.[11]
Gaseous N2O5 absorbs ultraviolet light with dissociation into the free radicals nitrogen dioxide NO2• and nitrogen trioxide NO3• (uncharged nitrate). The absorption spectrum has a broad band with maximum at wavelength 160 nm.[12]
Preparation
A recommended laboratory synthesis entails dehydrating nitric acid (HNO3) with phosphorus(V) oxide:[11]
- P4O10 + 12 HNO3 → 4 H3PO4 + 6 N2O5
Another laboratory process is the reaction of lithium nitrate LiNO3 and bromine pentafluoride BrF5, in the ratio exceeding 3:1. The reaction first forms nitryl fluoride FNO2 that reacts further with the lithium nitrate:[8]
- BrF5 + 3 LiNO3 → 3 LiF + BrONO2 + O2 + 2 FNO2
- FNO2 + LiNO3 → LiF + N2O5
The compound can also be created in the gas phase by reacting nitrogen dioxide NO2 or N2O4 with ozone:[13]
- 2 NO2 + O3 → N2O5 + O2
However, the product catalyzes the rapid decomposition of ozone:[13]
- 2 O3 + N2O5 → 3 O2 + N2O5
Dinitrogen pentoxide is also formed when a mixture of oxygen and nitrogen is passed through an electric discharge.[8] Another route is the reactions of Phosphoryl chloride POCl3 or nitryl chloride NO2Cl with silver nitrate AgNO3[8][14]
Reactions
Dinitrogen pentoxide reacts with water (hydrolyses) to produce nitric acid HNO3. Thus, dinitrogen pentoxide is the anhydride of nitric acid:[11]
- N2O5 + H2O → 2 HNO3
Solutions of dinitrogen pentoxide in nitric acid can be seen as nitric acid with more than 100% concentration. The phase diagram of the system H2O−N2O5 shows the well-known negative azeotrope at 60% N2O5 (that is, 70% HNO3), a positive azeotrope at 85.7% N2O5 (100% HNO3), and another negative one at 87.5% N2O5 ("102% HNO3").[15]
The reaction with hydrogen chloride HCl also gives nitric acid and nitryl chloride NO2Cl:[16]
- N2O5 + HCl → HNO3 + NO2Cl
Dinitrogen pentoxide eventually decomposes at room temperature into NO2 and O2.[17][13] Decomposition is negligible if the solid is kept at 0 °C, in suitably inert containers.[8]
Dinitrogen pentoxide reacts with ammonia NH3 to give several products, including nitrous oxide N2O, ammonium nitrate NH4NO3, nitramide NH2NO2 and ammonium dinitramide NH4N(NO2)2, depending on reaction conditions.[18]
Decomposition of dinitrogen pentoxide at high temperatures
Dinitrogen pentoxide between high temperatures of 600 and 1,100 K (327–827 °C), is decomposed in two successive stoichiometric steps:
- N2O5 → NO2 + NO3
- 2 NO3 → 2 NO2 + O2
In the shock wave, N2O5 has decomposed stoichiometrically into nitrogen dioxide and oxygen. At temperatures of 600 K and higher, nitrogen dioxide is unstable with respect to nitrogen oxide NO and oxygen. The thermal decomposition of 0.1 mM nitrogen dioxide at 1000 K is known to require about two seconds.[19]
Decomposition of dinitrogen pentoxide in carbon tetrachloride at 30 °C
Apart from the decomposition of N2O5 at high temperatures, it can also be decomposed in carbon tetrachloride CCl4 at 30 °C (303 K).[20] Both N2O5 and NO2 are soluble in CCl4 and remain in solution while oxygen is insoluble and escapes. The volume of the oxygen formed in the reaction can be measured in a gas burette. After this step we can proceed with the decomposition, measuring the quantity of O2 that is produced over time because the only form to obtain O2 is with the N2O5 decomposition. The equation below refers to the decomposition of N2O5 in CCl4:
- 2 N2O5 → 4 NO2 + O2(g)
And this reaction follows the first order rate law that says:
Decomposition of nitrogen pentoxide in the presence of nitric oxide
N2O5 can also be decomposed in the presence of nitric oxide NO:
- N2O5 + NO → 3 NO2
The rate of the initial reaction between dinitrogen pentoxide and nitric oxide of the elementary unimolecular decomposition.[21]
Applications
Nitration of organic compounds
Dinitrogen pentoxide, for example as a solution in chloroform, has been used as a reagent to introduce the −NO2 functionality in organic compounds. This nitration reaction is represented as follows:
- N2O5 + Ar−H → HNO3 + Ar−NO2
where Ar represents an arene moiety.[22] The reactivity of the NO+2 can be further enhanced with strong acids that generate the "super-electrophile" HNO2+2.
In this use, N2O5 has been largely replaced by nitronium tetrafluoroborate [NO2]+[BF4]−. This salt retains the high reactivity of NO+2, but it is thermally stable, decomposing at about 180 °C (into NO2F and BF3).
Dinitrogen pentoxide is relevant to the preparation of explosives.[7][23]
Atmospheric occurrence
In the atmosphere, dinitrogen pentoxide is an important reservoir of the NOx species that are responsible for ozone depletion: its formation provides a null cycle with which NO and NO2 are temporarily held in an unreactive state.[24] Mixing ratios of several parts per billion by volume have been observed in polluted regions of the nighttime troposphere.[25] Dinitrogen pentoxide has also been observed in the stratosphere[26] at similar levels, the reservoir formation having been postulated in considering the puzzling observations of a sudden drop in stratospheric NO2 levels above 50 °N, the so-called 'Noxon cliff'.
Variations in N2O5 reactivity in aerosols can result in significant losses in tropospheric ozone, hydroxyl radicals, and NOx concentrations.[27] Two important reactions of N2O5 in atmospheric aerosols are hydrolysis to form nitric acid[28] and reaction with halide ions, particularly Cl−, to form ClNO2 molecules which may serve as precursors to reactive chlorine atoms in the atmosphere.[29][30]
Hazards
N2O5 is a strong oxidizer that forms explosive mixtures with organic compounds and ammonium salts. The decomposition of dinitrogen pentoxide produces the highly toxic nitrogen dioxide gas.
References
- Haynes, p. 4.76
- Simon, Arndt; Horakh, Jörg; Obermeyer, Axel; Borrmann, Horst (1992). "Kristalline Stickstoffoxide — Struktur von N2O3 mit einer Anmerkung zur Struktur von N2O5". Angewandte Chemie (in German). Wiley. 104 (3): 325–327. Bibcode:1992AngCh.104..325S. doi:10.1002/ange.19921040321.
- Haynes, p. 5.29
- Connell, Peter Steele. (1979) The Photochemistry of Dinitrogen Pentoxide. Ph. D. thesis, Lawrence Berkeley National Laboratory.
- Angus, W.R.; Jones, R.W.; Phillips, G.O. (1949). "Existence of Nitrosyl Ions (NO+) in Dinitrogen Tetroxide and of Nitronium Ions (NO2+) in Liquid Dinitrogen Pentoxide". Nature. 164 (4167): 433. Bibcode:1949Natur.164..433A. doi:10.1038/164433a0. PMID 18140439. S2CID 4136455.
- Deville, M.H. (1849). "Note sur la production de l'acide nitrique anhydre". Compt. Rend. 28: 257–260.
- Agrawal, Jai Prakash (2010). High Energy Materials: Propellants, Explosives and Pyrotechnics. Wiley-VCH. p. 117. ISBN 978-3-527-32610-5. Retrieved 20 September 2011.
- Wilson, William W.; Christe, Karl O. (1987). "Dinitrogen pentoxide. New synthesis and laser Raman spectrum". Inorganic Chemistry. 26 (10): 1631–1633. doi:10.1021/ic00257a033.
- McDaniel, A. H.; Davidson, J. A.; Cantrell, C. A.; Shetter, R. E.; Calvert, J. G. (1988). "Enthalpies of formation of dinitrogen pentoxide and the nitrate free radical". The Journal of Physical Chemistry. 92 (14): 4172–4175. doi:10.1021/j100325a035.
- Parthiban, S.; Raghunandan, B.N.; Sumathi, R. (1996). "Structures, energies and vibrational frequencies of dinitrogen pentoxide". Journal of Molecular Structure: Theochem. 367: 111–118. doi:10.1016/S0166-1280(96)04516-2.
- Holleman, Arnold Frederik; Wiberg, Egon (2001), Wiberg, Nils (ed.), Inorganic Chemistry, translated by Eagleson, Mary; Brewer, William, San Diego/Berlin: Academic Press/De Gruyter, ISBN 0-12-352651-5
- Osborne, Bruce A.; Marston, George; Kaminski, L.; Jones, N.C; Gingell, J.M; Mason, Nigel; Walker, Isobel C.; Delwiche, J.; Hubin-Franskin, M.-J. (2000). "Vacuum ultraviolet spectrum of dinitrogen pentoxide". Journal of Quantitative Spectroscopy and Radiative Transfer. 64 (1): 67–74. Bibcode:2000JQSRT..64...67O. doi:10.1016/S0022-4073(99)00104-1.
- Yao, Francis; Wilson, Ivan; Johnston, Harold (1982). "Temperature-dependent ultraviolet absorption spectrum for dinitrogen pentoxide". The Journal of Physical Chemistry. 86 (18): 3611–3615. doi:10.1021/j100215a023.
- Schott, Garry; Davidson, Norman (1958). "Shock Waves in Chemical Kinetics: The Decomposition of N2O5 at High Temperatures". Journal of the American Chemical Society. 80 (8): 1841–1853. doi:10.1021/ja01541a019.
- Lloyd, L.; Wyatt, P. A. H. (1955). "The vapour pressures of nitric acid solutions. Part I. New azeotropes in the water–dinitrogen pentoxide system". J. Chem. Soc.: 2248–2252. doi:10.1039/JR9550002248.
- Wilkins, Robert A.; Hisatsune, I. C. (1976). "The Reaction of Dinitrogen Pentoxide with Hydrogen Chloride". Industrial & Engineering Chemistry Fundamentals. 15 (4): 246–248. doi:10.1021/i160060a003.
- Gruenhut, N. S.; Goldfrank, M.; Cushing, M. L.; Caesar, G. V.; Caesar, P. D.; Shoemaker, C. (1950). "Nitrogen(V) Oxide (Nitrogen Pentoxide, Dinitrogen Pentoxide, Nitric Anhydride)". Inorganic Syntheses. Inorganic Syntheses. pp. 78–81. doi:10.1002/9780470132340.ch20. ISBN 9780470132340.
- Frenck, C.; Weisweiler, W. (2002). "Modeling the Reactions Between Ammonia and Dinitrogen Pentoxide to Synthesize Ammonium Dinitramide (ADN)". Chemical Engineering & Technology. 25 (2): 123. doi:10.1002/1521-4125(200202)25:2<123::AID-CEAT123>3.0.CO;2-W.
- Schott, Garry; Davidson, Norman (1958). "Shock Waves in Chemical Kinetics: The Decomposition of N2O5 at High Temperatures". Journal of the American Chemical Society. 80 (8): 1841–1853. doi:10.1021/ja01541a019.
- Jaime, R. (2008). Determinación de orden de reacción haciendo uso de integrales definidas. Universidad Nacional Autónoma de Nicaragua, Managua.
- Wilson, David J.; Johnston, Harold S. (1953). "Decomposition of Nitrogen Pentoxide in the Presence of Nitric Oxide. IV. Effect of Noble Gases". Journal of the American Chemical Society. 75 (22): 5763. doi:10.1021/ja01118a529.
- Bakke, Jan M.; Hegbom, Ingrid; Verne, Hans Peter; Weidlein, Johann; Schnöckel, Hansgeorg; Paulsen, Gudrun B.; Nielsen, Ruby I.; Olsen, Carl E.; Pedersen, Christian; Stidsen, Carsten E. (1994). "Dinitrogen Pentoxide--Sulfur Dioxide, a New Nitration System". Acta Chemica Scandinavica. 48: 181–182. doi:10.3891/acta.chem.scand.48-0181.
- Talawar, M. B. (2005). "Establishment of Process Technology for the Manufacture of Dinitrogen Pentoxide and its Utility for the Synthesis of Most Powerful Explosive of Today—CL-20". Journal of Hazardous Materials. 124 (1–3): 153–64. doi:10.1016/j.jhazmat.2005.04.021. PMID 15979786.
- Finlayson-Pitts, Barbara J.; Pitts, James N. (2000). Chemistry of the upper and lower atmosphere : theory, experiments, and applications. San Diego: Academic Press. ISBN 9780080529073. OCLC 162128929.
- Wang, Haichao; Lu, Keding; Chen, Xiaorui; Zhu, Qindan; Chen, Qi; Guo, Song; Jiang, Meiqing; Li, Xin; Shang, Dongjie; Tan, Zhaofeng; Wu, Yusheng; Wu, Zhijun; Zou, Qi; Zheng, Yan; Zeng, Limin; Zhu, Tong; Hu, Min; Zhang, Yuanhang (2017). "High N2O5 Concentrations Observed in Urban Beijing: Implications of a Large Nitrate Formation Pathway". Environmental Science and Technology Letters. 4 (10): 416–420. doi:10.1021/acs.estlett.7b00341.
- Rinsland, C.P. (1989). "Stratospheric N2O5 profiles at sunrise and sunset from further analysis of the ATMOS/Spacelab 3 solar spectra". Journal of Geophysical Research. 94: 18341–18349. Bibcode:1989JGR....9418341R. doi:10.1029/JD094iD15p18341.
- Macintyre, H. L.; Evans, M. J. (2010-08-09). "Sensitivity of a global model to the uptake of N2O5 by tropospheric aerosol". Atmospheric Chemistry and Physics. 10 (15): 7409–7414. Bibcode:2010ACP....10.7409M. doi:10.5194/acp-10-7409-2010.
- Brown, S. S.; Dibb, J. E.; Stark, H.; Aldener, M.; Vozella, M.; Whitlow, S.; Williams, E. J.; Lerner, B. M.; Jakoubek, R. (2004-04-16). "Nighttime removal of NOx in the summer marine boundary layer". Geophysical Research Letters. 31 (7): n/a. Bibcode:2004GeoRL..31.7108B. doi:10.1029/2004GL019412.
- Gerber, R. Benny; Finlayson-Pitts, Barbara J.; Hammerich, Audrey Dell (2015-07-15). "Mechanism for formation of atmospheric Cl atom precursors in the reaction of dinitrogen oxides with HCl/Cl− on aqueous films" (PDF). Physical Chemistry Chemical Physics. 17 (29): 19360–19370. Bibcode:2015PCCP...1719360H. doi:10.1039/C5CP02664D. PMID 26140681. S2CID 39157816.
- Kelleher, Patrick J.; Menges, Fabian S.; DePalma, Joseph W.; Denton, Joanna K.; Johnson, Mark A.; Weddle, Gary H.; Hirshberg, Barak; Gerber, R. Benny (2017-09-18). "Trapping and Structural Characterization of the XNO2·NO3− (X = Cl, Br, I) Exit Channel Complexes in the Water-Mediated X− + N2O5 Reactions with Cryogenic Vibrational Spectroscopy". The Journal of Physical Chemistry Letters. 8 (19): 4710–4715. doi:10.1021/acs.jpclett.7b02120. PMID 28898581.
Cited sources
- Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. ISBN 9781498754293.
