Selective androgen receptor modulator
Selective androgen receptor modulators (SARMs) are a class of androgen receptor ligands that were developed with the intention of maintaining some of the desirable effects of androgens, such as improving bone density and increasing lean body mass, with a much lower risk of androgenic side effects than alternative therapies such as testosterone.
| Selective androgen receptor modulator | |
|---|---|
| Drug class | |
 Enobosarm (ostarine), a nonsteroidal SARM under investigation for potential medical use. | |
| Class identifiers | |
| Use | Investigational |
| Biological target | Androgen receptor |
| Chemical class | Steroidal; nonsteroidal |
| Legal status | |
| Legal status | |
| In Wikidata | |
Anabolic androgenic steroids (AAS) are potentially useful for a variety of medical conditions, but their use is limited by side effects. In the late 1990s, researchers discovered a new class of non-steroidal compounds chemically similar to the antiandrogens bicalutamide and hydroxyflutamide. These non-steroidal SARMs have a high anabolic activity in skeletal muscles, but have little or no androgenic activity in other tissues.
SARMs have been investigated in human studies for the treatment of osteoporosis, cachexia, benign prostatic hyperplasia, stress urinary incontinence, prostate cancer, and breast cancer and have also been considered for the treatment of Alzheimer’s disease, Duchenne muscular dystrophy, hypogonadism. and as a male contraceptive. As of 2020, there are no SARMs which have been approved for therapeutic use by the U.S. Food and Drug Administration. Although adverse effects in clinical studies have been infrequent and mild, SARMs can cause elevated liver enzymes, reduction of HDL cholesterol levels, and hypothalamic–pituitary–gonadal axis (HPG axis) suppression.
Since the early twenty-first century, SARMs have been used in doping; they were banned by the World Anti-Doping Agency in 2008. SARMs are readily available on internet-based gray markets and are commonly used recreationally to stimulate muscle growth.
Mechanism
Anabolic androgenic steroids (AAS), including those produced endogenously such as testosterone and dihydrotestosterone (DHT), bind to and activate the androgen receptor (AR) to produce their effects. AAS effects can be separated into androgenic (the development and maintenance of male sexual characteristics) and anabolic (increasing bone density, muscle mass and strength). AAS also affect hematopoiesis, coagulation, metabolism, and cognition.[2][3] Anti-androgens such as bicalutamide, flutamide, and nilutamide are non-steroidal AR antagonists that work by binding to the AR to prevent androgenic action; this class of chemicals dates to the 1970s.[2][4] Interest in non-steroidal AR agonists increased after the therapeutic uses of selective estrogen receptor modulators (SERMs) became evident.[4] The discovery of aryl propionamides, which share structural similarity with bicalutamide and hydroxyflutamide, suggested a way to make compounds that attach to the AR and produce both anabolic and anti-androgenic effects.[2] Selective androgen receptor modulators (SARMs) were developed out of a desire to maintain the anabolic effects of androgens on muscle and bone, while avoiding side effects on other tissues such as the prostate and cardiovascular system.[5]
The first SARMs to be discovered in the 1940s were steroidal[6] and formed by modifications to the testosterone molecule that change its binding affinity to the AR.[5] Non-steroidal SARMs were invented in 1998 independently by two research groups, one at the University of Tennessee that created an aryl propionamide SARM and Ligand Pharmaceuticals that made a SARM with a quinolone. The name was adopted by analogy with SERMs.[4] Other SARMs include tetrahydroquinolines, tricyclics, bridged tricyclics, aniline, diaryl aniline, bicylclic hydantoins, benzimidazole, imidazolopyrazole, indole, and pyrazoline derivatives.[2] SARMs can be agonists, antagonists, or partial agonists of the AR depending on the tissue, which can enable targeting specific medical conditions while minimizing side effects.[3] Those that have advanced to human trials show stronger effects in bone and muscle tissue and weaker effects in the prostate.[6] SARMs are orally bioavailable and largely eliminated via hepatic metabolism and metabolized through amide hydrolysis and A-ring nitro reduction.[5]
The mechanism of action of SARMs' tissue-specific effects continues to be debated as of 2020.[2][7] SARMs are not a substrate for 5a-reductase enzyme that converts testosterone to DHT, a highly androgenic compound. Other researchers argue that the differences in how SARMs attach to the androgen receptor compared to AAS account for the differences in their effect. In vitro testing of the SARMs ostarine and YK-11 showed that they bound to the AR, but unlike true AR agonists, blocked the amine and carboxy terminal. SARMs interact with transcription coregulators that are present in specific tissues as well as transcription factors, which plays a role in their selective effects.[2][8]
| Name | Class | Developer | Investigated for | Structure |
|---|---|---|---|---|
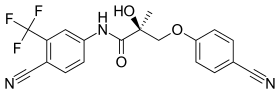
| Ostarine (Enobosarm) | Arylpropionamide | GTx | Breast cancer, cachexia, stress urinary incontinence |  |
| Andarine (S-4) | Arylpropionamide | GTx | Hepatocellular carcinoma[9] | 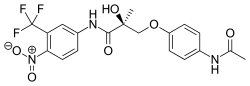 |
| GSK2881078 | GlaxoSmithKline | Muscle wasting[10] |  | |
| Ligandrol (LGD-4033) | Pyrrolidinebenzonitrile | Ligand Pharmaceuticals | Osteoporosis[11] | 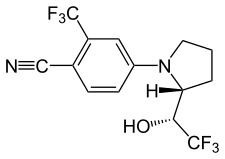 |
| LY305 | N-arylhydroxyalkyl | Eli Lilly | Osteoporosis[12] | 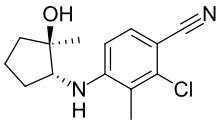 |
| OPK-88004 | Indole | OPKO | Benign prostatic hyperplasia, quality of life in prostate cancer patients[13] | 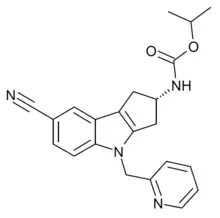 |
| RAD140 | Phenyloxadiazole | Radius Health | Breast cancer[14] | 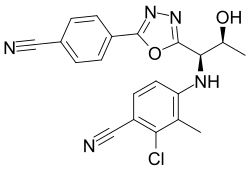 |
| YK-11 | Steroid | Muscle wasting[15] | 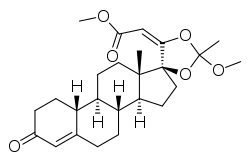
| |
Research and possible therapeutic applications
Due to their tissue selectivity, SARMs have the potential to treat a wide variety of conditions, including debilitating diseases. They have been investigated in human studies for the treatment of osteoporosis, cachexia, benign prostatic hyperplasia, stress urinary incontinence, prostate cancer, and breast cancer and have also been considered for the treatment of Alzheimer’s disease, Duchenne muscular dystrophy, hypogonadism and as a male contraceptive.[16][3] As of 2020, there are no SARMs which have been approved for therapeutic use by the U.S. Food and Drug Administration.[16]
Most SARMs have been tested in vitro or on rodents, while limited clinical trials in humans have been carried out.[2][17] Initial research focused on muscle wasting.[8] Ostarine is the most well-studied SARM; according to its manufacturer, GTx Incorporated, 25 studies have been carried out on more than 1,700 humans as of 2020 involving doses from 1 to 18 mg each day.[18][7] As of 2020, there is little research distinguishing different SARMs from each other.[2] Much of the research on SARMs has been conducted by corporations and has not been made publicly available.[6]
Benign prostatic hyperplasia
In rat models of BPH, SARMs reduced prostatic weight.[17] OPK-88004 advanced to a phase II trial in humans, but it was terminated due to difficulty in measuring prostate size, the trial's primary endpoint.[16]
Cancer
SARMs may help treat AR and estrogen receptor (ER) positive breast cancer, which comprise the majority of breast cancers.[3][19] AAS were historically used successfully to treat AR positive breast cancer, but were phased out after the development of anti-estrogen therapies, due to androgenic side effects and concerns about aromatization to estrogen (which does not occur with SARMs).[19][8] Although a trial on AR positive triple negative breast cancer (which is ER-) was ended early due to lack of efficacy, ostarine showed benefits in some patients with ER+, AR+ breast cancer in a phase II study. In patients with more than 40 percent AR positivity as determined by immunohistochemistry, the clinical benefit rate (CBR) was 80 percent and the objective response rate (ORR) was 48 percent—which was considered promising given that the patients had advanced disease and had been heavily pretreated.[20][19] In 2022, the FDA granted fast track designation to ostarine for AR+, ER+, HER2- metastatic breast cancer.[21] Other SARMs such as RAD140[14] and EP0062 have reached clinical trials in breast cancer patients.[22]
Bone and muscle wasting
As of 2020, there are no drugs approved to treat muscle wasting in people with chronic diseases, and there is therefore an unmet need for anabolic drugs with few side effects. One aspect hindering drug approval for treatments for cachexia and sarcopenia is disagreement in endpoints. Several clinical trials have found that SARMs improve lean mass in humans, but it is not clear whether strength and physical function are also improved. After promising results in a phase II trial, a phase III trial of ostarine was proven to increase lean body mass but did not show significant improvement in function. It and other drugs have been refused regulatory approval due to a lack of evidence that they increased physical performance; preventing decline in functionality was not considered an acceptable endpoint by the Food and Drug Administration. It is not known how SARMs interact with dietary protein intake and resistance training in people with muscle wasting.[7][16]
Phase II trials of ostarine for stress urinary incontinence—considered promising, given that the levator ani muscle involved has a high androgen receptor density—did not meet their endpoint and were abandoned.[16][8]
Unlike other treatments for osteoporosis, which work by decreasing bone loss, SARMs have shown potential to promote growth in bone tissue. LY305 showed promising results in a phase I trial in humans.[16]
Side effects
In contrast to AAS and testosterone replacement, which have many side effects that have curtailed their medical use, SARMs are well tolerated and have mild and infrequent adverse events in randomized controlled trials.[17] SARMs are not virilizing and cannot be aromatized to estrogen, thus causing no estrogenic side effects.[23][16][3] Unlike current versions of testosterone replacement, SARMs can be administered orally and do not have many drug interactions.[3]
SARM use can cause elevated liver enzymes and reduction in HDL cholesterol.[23][16] Transdermal administration may reduce these effects.[16] Although elevated liver enzymes caused by SARMs might indicate hepatocellular injury, it is not known if SARMs cause a significant risk of hepatoxicity.[17][3] Several case reports have associated SARMs with drug-induced liver injury when used recreationally.[24] Whether SARMs increase the risk of cardiovascular events is unknown.[17][3] SARMs have less effect on blood lipid profiles than testosterone replacement; it is not known whether androgen-induced HDL reductions increase cardiovascular risk; and SARMs increase insulin sensitivity and lower triglycerides.[3][7]
Although they cause less suppression of the hypothalamic–pituitary–gonadal axis (HPG axis) than testosterone, studies have found that gonadotropins, free and total testosterone, and SHBG can be reduced in a compound- and dose-dependent fashion in men from SARM usage.[2][7] Typically SHBG is reduced along with total testosterone and total cholesterol while hematocrit is increased. Most studies have found that follicle-stimulating hormone (FSH), lutenizing hormone (LH), prostate-specific antigen, estradiol, and DHT levels are not altered.[17] Of SARMs that have been investigated, ostarine is one of the least suppressive of gonadotropins, even in doses much higher than used in clinical trials. How the HPG axis is affected in women using SARMs is unknown.[2][7] SARMs' effect in suppressing the gonadotropins FSH and LH is what makes SARMs potentially useful as a male contraceptive.[25]
Non-medical use
Outside of pharmaceutical research, SARMs are a gray market substance produced by small laboratories and often marketed as a research chemical supposedly not for human consumption.[2][26][27] Marketing SARMs for human consumption is illegal in some jurisdictions and has led to criminal convictions in the United States[28] and the largest-ever fine levied under Australia's Therapeutic Goods Act 1989.[29] Although SARMs are readily available for purchase on the internet, one study found that a majority of products advertised as SARMs online were mislabeled. Anecdotes and guides on usage can also be found online and on social media.[30][23][3] Some compounds are commonly marketed for recreational use as SARMs despite having a different mechanism of action, including MK-677 / Ibutamoren (a growth hormone secretagogue), GW501516 / cardarine (an agonist of the PPARß/δ), and SR9009 / Stenabolic (an agonist of the Rev-Erb).[2]
SARMs are used by bodybuilders and competitive athletes due to their anabolic and lack of androgenic effects,[3] particularly in the United States, Europe, and other western countries.[23] Some individuals using SARMs recreationally combine multiple SARMs or take a SARM along with other compounds, although there is no research on combining SARMs. The doses used often exceed those from clinical trials; nevertheless, the fat-free mass gained from SARMs is generally lower than what is obtained with moderate doses of testosterone derivatives.[2] According to one study of SARM users, more than 90 percent were satisfied with their usage and 64.2 percent would take SARMS again even though a majority experienced adverse effects.[31]
SARMs were banned by the World Anti-Doping Agency (WADA) in 2008.[2] SARMs can be detected in urine and hair after consumption.[32] WADA reported its first adverse analytical finding for SARMs in 2010 and the number of positive tests has increased since then; the most commonly detected SARMs are ostarine and ligandrol.[33][34] Athletes competing in the NFL, NBA, UFC, NCAA, and the Olympics have tested positive.[24] There is limited evidence on how SARMs affect athletic performance.[35]
References
- Koh, Benjamin (22 March 2013). "Anti-doping agency warns cheats on the health risks of Endurobol". The Conversation. Retrieved 4 September 2023.
- Machek, Steven B.; Cardaci, Thomas D.; Wilburn, Dylan T.; Willoughby, Darryn S. (2020). "Considerations, possible contraindications, and potential mechanisms for deleterious effect in recreational and athletic use of selective androgen receptor modulators (SARMs) in lieu of anabolic androgenic steroids: A narrative review". Steroids. 164: 108753. doi:10.1016/j.steroids.2020.108753. ISSN 0039-128X. PMID 33148520. S2CID 225049089.
- Solomon, Zachary J.; Mirabal, Jorge Rivera; Mazur, Daniel J.; Kohn, Taylor P.; Lipshultz, Larry I.; Pastuszak, Alexander W. (2019). "Selective Androgen Receptor Modulators: Current Knowledge and Clinical Applications". Sexual Medicine Reviews. 7 (1): 84–94. doi:10.1016/j.sxmr.2018.09.006. PMC 6326857. PMID 30503797.
- Temerdashev, A. Z.; Dmitrieva, E. V. (2020). "Methods for the Determination of Selective Androgen Receptor Modulators". Journal of Analytical Chemistry. 75 (7): 835–850. doi:10.1134/S1061934820070187. S2CID 220398030.
- Bhasin, Shalender; Krishnan, Venkatesh; Storer, Thomas W; Steiner, Mitchell; Dobs, Adrian S (2023). "Androgens and Selective Androgen Receptor Modulators to Treat Functional Limitations Associated With Aging and Chronic Disease". The Journals of Gerontology: Series A. 78 (Supplement_1): 25–31. doi:10.1093/gerona/glad027. PMC 10272983. PMID 37325955.
- Jasuja, Ravi; Zacharov, Mikhail N.; Bhasin, Shalender (2012). "The state-of-the-art in the development of selective androgen receptor modulators". Testosterone: Action, Deficiency, Substitution (4 ed.). Cambridge University Press. pp. 459–460. ISBN 978-1-107-01290-5.
- Fonseca, Guilherme Wesley Peixoto Da; Dworatzek, Elke; Ebner, Nicole; Von Haehling, Stephan (2020). "Selective androgen receptor modulators (SARMs) as pharmacological treatment for muscle wasting in ongoing clinical trials". Expert Opinion on Investigational Drugs. 29 (8): 881–891. doi:10.1080/13543784.2020.1777275. PMID 32476495. S2CID 219174372.
- Narayanan, Ramesh; Coss, Christopher C.; Dalton, James T. (2018). "Development of selective androgen receptor modulators (SARMs)". Molecular and Cellular Endocrinology. 465: 134–142. doi:10.1016/j.mce.2017.06.013. PMC 5896569. PMID 28624515.
- Yavuz, Mervenur; Takanlou, Leila Sabour; Avcı, Çığır Biray; Demircan, Turan (2023). "A selective androgen receptor modulator, S4, displays robust anti-cancer activity on hepatocellular cancer cells by negatively regulating PI3K/AKT/mTOR signalling pathway". Gene. 869: 147390. doi:10.1016/j.gene.2023.147390. ISSN 0378-1119.
- Mohan, Divya; Rossiter, Harry; Watz, Henrik; Fogarty, Charles; Evans, Rachael A.; Man, William; Tabberer, Maggie; Beerahee, Misba; Kumar, Subramanya; Millns, Helen; Thomas, Sebin; Tal-Singer, Ruth; Russell, Alan J.; Holland, Marie Claire; Akinseye, Chika; Neil, David; Polkey, Michael I. (1 March 2023). "Selective androgen receptor modulation for muscle weakness in chronic obstructive pulmonary disease: a randomised control trial". Thorax. 78 (3): 258–266. doi:10.1136/thorax-2021-218360. ISSN 0040-6376. PMC 9985744. PMID 36283827.
- Hoffmann, Daniel B.; Derout, Christoph; Müller-Reiter, Max; Böker, Kai O.; Schilling, Arndt F.; Roch, Paul J.; Lehmann, Wolfgang; Saul, Dominik; Hawellek, Thelonius; Taudien, Stefan; Sehmisch, Stephan; Komrakova, Marina (2023). "Effects of ligandrol as a selective androgen receptor modulator in a rat model for osteoporosis". Journal of Bone and Mineral Metabolism. doi:10.1007/s00774-023-01453-8. ISSN 1435-5604.
- Krishnan, V.; Patel, N. J.; Mackrell, J. G.; Sweetana, S. A.; Bullock, H.; Ma, Y. L.; Waterhouse, T. H.; Yaden, B. C.; Henck, J.; Zeng, Q. Q.; Gavardinas, K.; Jadhav, P.; Saeed, A.; Garcia-Losada, P.; Robins, D. A.; Benson, C. T. (2018). "Development of a selective androgen receptor modulator for transdermal use in hypogonadal patients". Andrology. 6 (3): 455–464. doi:10.1111/andr.12479. PMID 29527831. S2CID 3858281.
- Pencina, Karol M; Burnett, Arthur L; Storer, Thomas W; Guo, Wen; Li, Zhuoying; Kibel, Adam S; Huang, Grace; Blouin, Michelle; Berry, Donna L; Basaria, Shehzad; Bhasin, Shalender (2021). "A Selective Androgen Receptor Modulator (OPK-88004) in Prostate Cancer Survivors: A Randomized Trial". The Journal of Clinical Endocrinology & Metabolism. 106 (8): 2171–2186. doi:10.1210/clinem/dgab361. PMC 8277210. PMID 34019661.
- Hamilton, Erika; LoRusso, Patricia; Ma, Cynthia; Vidula, Neelima; Bagley, Rebecca G; Troy, Steven; Annett, Miriam; Yu, Ziyang; Weitzman, Aaron; Conlan, Maureen G; Weise, Amy (15 February 2020). "Abstract P5-11-01 : Phase 1 dose escalation study of a novel selective androgen receptor modulator (SARM), RAD140, in estrogen receptor positive (ER+), human epidermal growth factor receptor 2 negative (HER2-), metastatic breast cancer". Cancer Research. 80 (4_Supplement): P5–11–01-P5-11-01. doi:10.1158/1538-7445.sabcs19-p5-11-01.
- Lee, Su Jin; Gharbi, Amal; Shin, Joo Eun; Jung, In Duk; Park, Yeong Min (2021). "Myostatin inhibitor YK11 as a preventative health supplement for bacterial sepsis". Biochemical and Biophysical Research Communications. 543: 1–7. doi:10.1016/j.bbrc.2021.01.030. ISSN 0006-291X. PMID 33588136. S2CID 231938058.
- Christiansen, Andrew R.; Lipshultz, Larry I.; Hotaling, James M.; Pastuszak, Alexander W. (March 2020). "Selective androgen receptor modulators: the future of androgen therapy?". Translational Andrology and Urology. 9 (Suppl 2): S135–S148. doi:10.21037/tau.2019.11.02. ISSN 2223-4683. PMC 7108998. PMID 32257854.
- Sigalos, John T.; Walker, Dyvon T.; Lipschultz, Larry I. (2023). "Selective Androgen Receptor Modulators in the Treatment of Hypogonadism and Men's Health". Men's Reproductive and Sexual Health Throughout the Lifespan: An Integrated Approach to Fertility, Sexual Function, and Vitality. Cambridge University Press. p. 266. ISBN 978-1-009-19755-7.
- Zajac, Jeffrey D.; Seeman, Ego; Russell, Nicholas; Ramchand, Sabashini K.; Bretherton, Ingrid; Grossmann, Mathis; Davey, Rachel A. (2020). "Testosterone". Encyclopedia of Bone Biology. Academic Press. p. 545. ISBN 978-0-12-814082-6.
- Dai, Charles; Ellisen, Leif W (2023). "Revisiting Androgen Receptor Signaling in Breast Cancer". The Oncologist. 28 (5): 383–391. doi:10.1093/oncolo/oyad049. PMC 10166165. PMID 36972361.
- Palmieri, Carlo; Linden, Hannah M.; Birrell, Stephen; Lim, Elgene; Schwartzberg, Lee S.; Rugo, Hope S.; Cobb, Patrick Wayne; Jain, Kirti; Vogel, Charles L.; O'Shaughnessy, Joyce; Johnston, Stephen R. D.; Getzenberg, Robert H.; Barnette, K. Gary; Steiner, Mitchell S.; Brufsky, Adam; Overmoyer, Beth (2021). "Efficacy of enobosarm, a selective androgen receptor (AR) targeting agent, correlates with the degree of AR positivity in advanced AR+/estrogen receptor (ER)+ breast cancer in an international phase 2 clinical study". Journal of Clinical Oncology. 39 (15_suppl): 1020. doi:10.1200/JCO.2021.39.15_suppl.1020. ISSN 0732-183X. S2CID 236407030.
- "FDA Grants Fast Track Designation to Enobosarm in AR+, ER+, HER2- Metastatic Breast Cancer". Cancer Network. 10 January 2022. Retrieved 27 August 2023.
- Lim, Elgene; Hamilton, Erika; Palmieri, Carlo; Arkenau, Hendrik-Tobias; Brook, Sue; Fisher, Geoff; Mazur, Andrew (1 March 2023). "Abstract OT1-02-02: A phase 1/2 study to evaluate the safety and efficacy of EP0062, an oral Selective Androgen Receptor Modulator (SARM), for the treatment of AR+/HER2-/ER+ advanced breast cancer". Cancer Research. 83 (5_Supplement): OT1–02–02-OT1-02-02. doi:10.1158/1538-7445.SABCS22-OT1-02-02.
- Xie, Youquan; Tian, Yucheng; Zhang, Yuming; Zhang, Zhisheng; Chen, Rui; Li, Mian; Tang, Jiawei; Bian, Jinlei; Li, Zhiyu; Xu, Xi (15 February 2022). "Overview of the development of selective androgen receptor modulators (SARMs) as pharmacological treatment for osteoporosis (1998–2021)". European Journal of Medicinal Chemistry. 230: 114119. doi:10.1016/j.ejmech.2022.114119. ISSN 0223-5234. PMID 35063736. S2CID 245941791.
- Hahamyan, Henrik; Gould, Heath; Gregory, Andrew; Dodson, Christopher; Gausden, Elizabeth; Voos, James; Calcei, Jacob; Vasireddi, Nikhil (2023). "Poster 390: Systematic Review of SARMs Abuse in Athletes". Orthopaedic Journal of Sports Medicine. 11 (7_suppl3). doi:10.1177/2325967123S00352. ISSN 2325-9671. S2CID 260375399.
- Bhasin, Shalender (2015). "Selective Androgen Receptor Modulators as Function Promoting Therapies". The Journal of Frailty & Aging. 4 (3): 121–122. doi:10.14283/jfa.2015.65. ISSN 2260-1341. PMC 6039107. PMID 27030938.
- Sobolevsky, Tim; Ahrens, Brian (2021). "High‐throughput liquid chromatography tandem mass spectrometry assay as initial testing procedure for analysis of total urinary fraction". Drug Testing and Analysis. 13 (2): 283–298. doi:10.1002/dta.2917. ISSN 1942-7603. PMID 32852861. S2CID 221347916.
- Turnock, Dr Luke; Gibbs, Dr Nick (2023). "Click, click, buy: The market for novel synthetic peptide hormones on mainstream e-commerce platforms in the UK". Performance Enhancement & Health. 11 (2): 100251. doi:10.1016/j.peh.2023.100251. ISSN 2211-2669. S2CID 257706930.
- Oberheiden, Nick (26 June 2023). "The FDA Continues to Crack Down on SARM Manufacturing and Distribution". Federal Lawyer. Retrieved 13 October 2023.
- Jacobson, Hogan Lovells-Mandi; Zhang, Angell; Forrai, Zachary (3 August 2021). "Failure to remove unlawful advertising attracts $12 million penalty". Lexology. Retrieved 13 October 2023.
- Hahamyan, Henrik A.; Vasireddi, Nikhil; Voos, James E.; Calcei, Jacob G. (2023). "Social media's impact on widespread SARMs abuse". The Physician and Sportsmedicine. 51 (4): 291–293. doi:10.1080/00913847.2022.2078679. ISSN 0091-3847. PMID 35574698. S2CID 248812455.
- Efimenko, Iakov V.; Valancy, David; Dubin, Justin M.; Ramasamy, Ranjith (2022). "Adverse effects and potential benefits among selective androgen receptor modulators users: a cross-sectional survey". International Journal of Impotence Research. 34 (8): 757–761. doi:10.1038/s41443-021-00465-0. ISSN 1476-5489. PMID 34471228. S2CID 237378326.
- Kintz, Pascal; Ameline, Alice; Gheddar, Laurie; Raul, Jean-Sébastien (2019). "LGD-4033, S-4 and MK-2866 – Testing for SARMs in hair: About 2 doping cases". Toxicologie Analytique et Clinique. 31 (1): 56–63. doi:10.1016/j.toxac.2018.12.001. ISSN 2352-0078.
- "Selektive Androgenrezeptor-Modulatoren (SARMs)". Institut für Biochemie, Deutsche Sporthochschule Köln (in German). Retrieved 1 September 2023.
- Kintz, Pascal (5 January 2022). "The forensic response after an adverse analytical finding (doping) involving a selective androgen receptor modulator (SARM) in human athlete". Journal of Pharmaceutical and Biomedical Analysis. 207: 114433. doi:10.1016/j.jpba.2021.114433. ISSN 1873-264X. PMID 34715583. S2CID 240229684.
- Warrier, Alec A.; Azua, Eric N.; Kasson, Luke B.; Allahabadi, Sachin; Khan, Zeeshan A.; Mameri, Enzo S.; Swindell, Hasani W.; Tokish, John M.; Chahla, Jorge (2023). "Performance-Enhancing Drugs in Healthy Athletes: An Umbrella Review of Systematic Reviews and Meta-analyses". Sports Health: A Multidisciplinary Approach. doi:10.1177/19417381231197389. ISSN 1941-7381.