Sodium amide
Sodium amide, commonly called sodamide (systematic name sodium azanide), is the inorganic compound with the formula NaNH2. It is a salt composed of the sodium cation and the azanide anion. This solid, which is dangerously reactive toward water, is white, but commercial samples are typically gray due to the presence of small quantities of metallic iron from the manufacturing process. Such impurities do not usually affect the utility of the reagent. NaNH2 conducts electricity in the fused state, its conductance being similar to that of NaOH in a similar state. NaNH2 has been widely employed as a strong base in organic synthesis.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Sodium amide, sodium azanide[1] | |
| Other names
Sodamide | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.029.064 |
| EC Number |
|
PubChem CID |
|
| UNII | |
| UN number | 1390 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| NaNH2 | |
| Molar mass | 39.013 g·mol−1 |
| Appearance | Colourless crystals |
| Odor | Ammonia-like |
| Density | 1.39 g/cm3 |
| Melting point | 210 °C (410 °F; 483 K) |
| Boiling point | 400 °C (752 °F; 673 K) |
| Reacts | |
| Solubility | 40 mg/L (liquid ammonia), reacts with ethanol |
| Acidity (pKa) | 38 (conjugate acid)[2] |
| Structure | |
| orthorhombic | |
| Thermochemistry | |
Heat capacity (C) |
66.15 J/(mol·K) |
Std molar entropy (S⦵298) |
76.9 J/(mol·K) |
Std enthalpy of formation (ΔfH⦵298) |
-118.8 kJ/mol |
Gibbs free energy (ΔfG⦵) |
-59 kJ/mol |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 4.44 °C (39.99 °F; 277.59 K) |
| 450 °C (842 °F; 723 K) | |
| Related compounds | |
Other anions |
Sodium bis(trimethylsilyl)amide |
Other cations |
Lithium amide Potassium amide |
Related compounds |
Ammonia |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
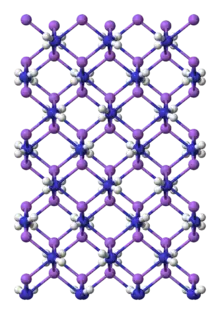
Preparation and structure
Sodium amide can be prepared by the reaction of sodium with ammonia gas,[3] but it is usually prepared by the reaction in liquid ammonia using iron(III) nitrate as a catalyst. The reaction is fastest at the boiling point of the ammonia, c. −33 °C. An electride, [Na(NH3)6]+e−, is formed as a reaction intermediate.[4]
- 2 Na + 2 NH3 → 2 NaNH2 + H2
NaNH2 is a salt-like material and as such, crystallizes as an infinite polymer.[5] The geometry about sodium is tetrahedral.[6] In ammonia, NaNH2 forms conductive solutions, consistent with the presence of [Na(NH3)6]+ and NH−2 ions.
Uses
Sodium amide is mainly used as a strong base in organic chemistry, often in liquid ammonia solution. It is the reagent of choice for the drying of ammonia (liquid or gaseous). One of the main advantages to the use of sodium amide is its relatively low nucleophilicity. In the industrial production of indigo, sodium amide is a component of the highly basic mixture that induces cyclisation of N-phenylglycine. The reaction produces ammonia, which is recycled typically.[7]
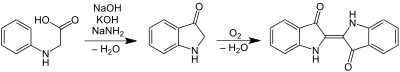
Dehydrohalogenation
Sodium amide induces the loss of two equivalents of hydrogen bromide from a vicinal dibromoalkane to give a carbon–carbon triple bond, as in a preparation of phenylacetylene.[8] Usually two equivalents of sodium amide yields the desired alkyne. Three equivalents are necessary in the preparation of a terminal alkynes because the terminal CH of the resulting alkyne protonates an equivalent amount of base.

Hydrogen chloride and ethanol can also be eliminated in this way,[9] as in the preparation of 1-ethoxy-1-butyne.[10]
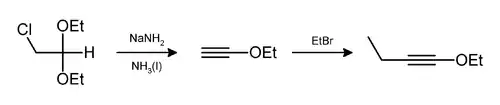
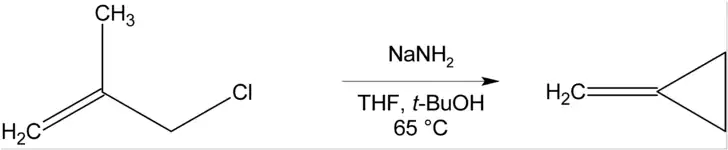
Cyclization reactions
Where there is no β-hydrogen to be eliminated, cyclic compounds may be formed, as in the preparation of methylenecyclopropane below.[11]
Cyclopropenes,[12] aziridines[13] and cyclobutanes[14] may be formed in a similar manner.
Deprotonation of carbon and nitrogen acids
Carbon acids which can be deprotonated by sodium amide in liquid ammonia include terminal alkynes,[15] methyl ketones,[16] cyclohexanone,[17] phenylacetic acid and its derivatives[18] and diphenylmethane.[19] Acetylacetone loses two protons to form a dianion.[20] Sodium amide will also deprotonate indole[21] and piperidine.[22]
Related non-nucleophilic bases
It is however poorly soluble in solvents other than ammonia. Its use has been superseded by the related reagents sodium hydride, sodium bis(trimethylsilyl)amide (NaHMDS), and lithium diisopropylamide (LDA).
Other reactions
- Rearrangement with orthodeprotonation[23]
- Oxirane synthesis[24]
- Indole synthesis[25]
- Chichibabin reaction
Safety
Sodium amide decomposes violently on contact with water, producing ammonia and sodium hydroxide:
- NaNH2 + H2O → NH3 + NaOH
When burned in oxygen, it will give oxides of sodium (which react with the produced water, giving sodium hydroxide) along with nitrogen oxides:
- 4 NaNH2 + 5 O2 → 4 NaOH + 4 NO + 2 H2O
- 4 NaNH2 + 7 O2 → 4 NaOH + 4 NO2 + 2 H2O
In the presence of limited quantities of air and moisture, such as in a poorly closed container, explosive mixtures of peroxides may form.[26] This is accompanied by a yellowing or browning of the solid. As such, sodium amide is to be stored in a tightly closed container, under an atmosphere of an inert gas. Sodium amide samples which are yellow or brown in color represent explosion risks.[27]
References
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "amides". doi:10.1351/goldbook.A00266
- Buncel, E.; Menon, B. (1977). "Carbanion mechanisms: VII. Metallation of hydrocarbon acids by potassium amide and potassium methylamide in tetrahydrofuran and the relative hydride acidities". Journal of Organometallic Chemistry. 141 (1): 1–7. doi:10.1016/S0022-328X(00)90661-2.
- Bergstrom, F. W. (1955). "Sodium amide". Organic Syntheses.; Collective Volume, vol. 3, p. 778
- Greenlee, K. W.; Henne, A. L. (1946). "Sodium Amide". Inorganic Syntheses. Inorganic Syntheses. Vol. 2. pp. 128–135. doi:10.1002/9780470132333.ch38. ISBN 9780470132333.
- Zalkin, A.; Templeton, D. H. (1956). "The Crystal Structure Of Sodium Amide". Journal of Physical Chemistry. 60 (6): 821–823. doi:10.1021/j150540a042. hdl:2027/mdp.39015086484659.
- Wells, A. F. (1984). Structural Inorganic Chemistry. Oxford: Clarendon Press. ISBN 0-19-855370-6.
- L. Lange, W. Treibel "Sodium Amide" in Ullmann's Encyclopedia of Industrial Chemistry 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a24_267
- Campbell, K. N.; Campbell, B. K. (1950). "Phenylacetylene". Organic Syntheses. 30: 72.
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collective Volume, vol. 4, p. 763 - Jones, E. R. H.; Eglinton, G.; Whiting, M. C.; Shaw, B. L. (1954). "Ethoxyacetylene". Organic Syntheses. 34: 46.
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collective Volume, vol. 4, p. 404
Bou, A.; Pericàs, M. A.; Riera, A.; Serratosa, F. (1987). "Dialkoxyacetylenes: di-tert-butoxyethyne, a valuable synthetic intermediate". Organic Syntheses. 65: 58.{{cite journal}}: CS1 maint: multiple names: authors list (link); Collective Volume, vol. 8, p. 161
Magriotis, P. A.; Brown, J. T. (1995). "Phenylthioacetylene". Organic Syntheses. 72: 252.{{cite journal}}: CS1 maint: multiple names: authors list (link); Collective Volume, vol. 9, p. 656
Ashworth, P. J.; Mansfield, G. H.; Whiting, M. C. (1955). "2-Butyn-1-ol". Organic Syntheses. 35: 20.{{cite journal}}: CS1 maint: multiple names: authors list (link); Collective Volume, vol. 4, p. 128 - Newman, M. S.; Stalick, W. M. (1977). "1-Ethoxy-1-butyne". Organic Syntheses. 57: 65.
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collective Volume, vol. 6, p. 564 - Salaun, J. R.; Champion, J.; Conia, J. M. (1977). "Cyclobutanone from methylenecyclopropane via oxaspiropentane". Organic Syntheses. 57: 36.
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collective Volume, vol. 6, p. 320 - Nakamura, M.; Wang, X. Q.; Isaka, M.; Yamago, S.; Nakamura, E. (2003). "Synthesis and (3+2)-cycloaddition of a 2,2-dialkoxy-1-methylenecyclopropane: 6,6-dimethyl-1-methylene-4,8-dioxaspiro(2.5)octane and cis-5-(5,5-dimethyl-1,3-dioxan-2-ylidene)hexahydro-1(2H)-pentalen-2-one". Organic Syntheses. 80: 144.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Bottini, A. T.; Olsen, R. E. (1964). "N-Ethylallenimine". Organic Syntheses. 44: 53.
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collective Volume, vol. 5, p. 541 - Skorcz, J. A.; Kaminski, F. E. (1968). "1-Cyanobenzocyclobutene". Organic Syntheses. 48: 55.
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collective Volume, vol. 5, p. 263 - Saunders, J. H. (1949). "1-Ethynylcyclohexanol". Organic Syntheses. 29: 47.; Collective Volume, vol. 3, p. 416
Peterson, P. E.; Dunham, M. (1977). "(Z)-4-Chloro-4-hexenyl trifluoroacetate". Organic Syntheses. 57: 26.{{cite journal}}: CS1 maint: multiple names: authors list (link); Collective Volume, vol. 6, p. 273
Kauer, J. C.; Brown, M. (1962). "Tetrolic acid". Organic Syntheses. 42: 97.{{cite journal}}: CS1 maint: multiple names: authors list (link); Collective Volume, vol. 5, p. 1043 - Coffman, D. D. (1940). "Dimethylethynylcarbinol". Organic Syntheses. 20: 40.; Collective Volume, vol. 3, p. 320Hauser, C. R.; Adams, J. T.; Levine, R. (1948). "Diisovalerylmethane". Organic Syntheses. 28: 44.
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collective Volume, vol. 3, p. 291 - Vanderwerf, C. A.; Lemmerman, L. V. (1948). "2-Allylcyclohexanone". Organic Syntheses. 28: 8.
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collective Volume, vol. 3, p. 44 - Hauser, C. R.; Dunnavant, W. R. (1960). "α,β-Diphenylpropionic acid". Organic Syntheses. 40: 38.
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collective Volume, vol. 5, p. 526
Kaiser, E. M.; Kenyon, W. G.; Hauser, C. R. (1967). "Ethyl 2,4-diphenylbutanoate". Organic Syntheses. 47: 72.{{cite journal}}: CS1 maint: multiple names: authors list (link); Collective Volume, vol. 5, p. 559
Wawzonek, S.; Smolin, E. M. (1951). "α,β-Diphenylcinnamonitrile". Organic Syntheses. 31: 52.{{cite journal}}: CS1 maint: multiple names: authors list (link); Collective Volume, vol. 4, p. 387 - Murphy, W. S.; Hamrick, P. J.; Hauser, C. R. (1968). "1,1-Diphenylpentane". Organic Syntheses. 48: 80.
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collective Volume, vol. 5, p. 523 - Hampton, K. G.; Harris, T. M.; Hauser, C. R. (1971). "Phenylation of diphenyliodonium chloride: 1-phenyl-2,4-pentanedione". Organic Syntheses. 51: 128.
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collective Volume, vol. 6, p. 928
Hampton, K. G.; Harris, T. M.; Hauser, C. R. (1967). "2,4-Nonanedione". Organic Syntheses. 47: 92.{{cite journal}}: CS1 maint: multiple names: authors list (link); Collective Volume, vol. 5, p. 848 - Potts, K. T.; Saxton, J. E. (1960). "1-Methylindole". Organic Syntheses. 40: 68.
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collective Volume, vol. 5, p. 769 - Bunnett, J. F.; Brotherton, T. K.; Williamson, S. M. (1960). "N-β-Naphthylpiperidine". Organic Syntheses. 40: 74.
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collective Volume, vol. 5, p. 816 - Brazen, W. R.; Hauser, C. R. (1954). "2-Methylbenzyldimethylamine". Organic Syntheses. 34: 61.
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collective Volume, vol. 4, p. 585 - Allen, C. F. H.; VanAllan, J. (1944). "Phenylmethylglycidic ester". Organic Syntheses. 24: 82.
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collective Volume, vol. 3, p. 727 - Allen, C. F. H.; VanAllan, J. (1942). "2-Methylindole". Organic Syntheses. 22: 94.
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collective Volume, vol. 3, p. 597 - Clark, Donald E (2001). "Peroxides and peroxide-forming compounds". Chemical Health and Safety. 8 (5): 12–22. doi:10.1016/S1074-9098(01)00247-7. ISSN 1074-9098.
- "Sodium amide SOP". Princeton.
