Teloschistaceae
The Teloschistaceae are a large family of mostly lichen-forming fungi belonging to the class Lecanoromycetes in the division Ascomycota. Many members of the Teloschistaceae are readily identifiable by their vibrant orange to yellow hue, a result of its frequent anthraquinone content. The presence of these anthraquinone pigments, which confer protection from ultraviolet light, enabled this group to expand from shaded forest habitats to harsher environmental conditions of sunny and arid ecosystems during the Late Cretaceous. Collectively, the family has a cosmopolitan distribution, although members occur predominantly in subtropical and temperate regions. Although most members either live on rock or on bark, about 40 species are lichenicolous–meaning they live on other lichens.
| Teloschistaceae | |
|---|---|
.jpg.webp) | |
| Teloschistes flavicans is the type species of the type genus of the family Teloschistaceae. | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Fungi |
| Division: | Ascomycota |
| Class: | Lecanoromycetes |
| Order: | Teloschistales |
| Family: | Teloschistaceae Zahlbr. (1898) |
| Type genus | |
| Teloschistes Norman (1853) | |
| Subfamilies | |
|
Caloplacoideae – 37 genera Teloschistoideae – 33 genera Xanthorioideae – 45 genera | |
| Synonyms[1] | |
| |
Teloschistaceae lichens encompass a diverse array of physical forms, although the thallus (the main body of the lichen) is either leaf-like (foliose, bushy or shrub-like (fruticose) to crust-like. These lichens typically partner with a photosynthetic companion (a photobiont) from the green algal genus Trebouxia or similar genera. Teloschistaceae members are also characterized by their apothecia (the fruiting bodies where sexual reproduction occurs), which generally have well-defined thalline margins, contributing to the lichen's overall structure and appearance. A notable feature of Teloschistaceae is the bluish reaction of the ascus tip's external layer to iodine – the ascus being the spore-producing structure. The ascospores are released through a longitudinal slit in the tip of the ascus, a unique characteristic common to this group of lichens.
The family, first formally proposed in 1898, was extensively revised in 2013, including the recognition of three distinct subfamilies (Caloplacoideae, Teloschistoideae, and Xanthorioideae), and the creation or resurrection of 31 genera. Several dozen new genera have been added, though not without some controversy, as ongoing molecular studies provide clearer insights into the phylogenetic relationships amongst taxa within this family. Depending on the source consulted, the family is estimated to contain up to more than 1000 species and 117 genera. Three Teloschistaceae species have been assessed for the global IUCN Red List.
Systematics
Historical taxonomy
The first members of the present-day Teloschistaceae to be formally described were the common sunburst lichen (Xanthoria parietina) and the gold-eye lichen (Teloschistes chrysophthalmus). These were two of several dozen lichen species described by Swedish taxonomist Carl Linnaeus, the former in his influential 1753 treatise Species Plantarum, and the latter in his 1771 work Mantissa Plantarum II.[2]
In his 1852 work Synopsis Lichenum Blasteniosporum ("Synopsis of Lichen Blasteniospores"),[3] Italian lichenologist Abramo Bartolommeo Massalongo attempted to classify what he called "blasteniospore lichens". This referred to species with widely different growth forms and appearance that shared the typical polar-diblastic spores now recognised as the family Teloschistaceae. These are spores that are divided into two compartments (locules) separated by a central septum with a perforation. Although Massalongo's efforts to arrange these taxa into more natural genera were largely ignored by later contemporaries, several of his proposed genera were resurrected for use 16 decades later, such as Blastenia, Gyalolechia, Pyrenodesmia, and Xanthocarpia.[4]
The family Teloschistaceae was formally circumscribed by lichenologist Alexander Zahlbruckner in 1898. In his initial version, he grouped together foliose and fruticose taxa having polarilocular or 4-locule ascospores, including the genera Xanthoria, Teloschistes, and Lethariopsis.[5] At that time, the growth form of the lichen thalli was often used in classical lichen taxonomy to segregate groups of species into families,[6] and so in a subsequent (1926) publication, Zahlbruckner introduced the family Caloplacaceae to contain crustose lichens with polarilocular ascospores (rarely simple or with 3 or 4 locules); this family included the genera Caloplaca, Blastenia, Bombyliospora, and Protoblastenia.[7] However, the distinctness of the family Caloplacaceae was largely rejected by other authors,[8] and it is now a historical synonym of Teloschistaceae.[1] In another older classification, crustose genera were grouped together in the family Blasteniaceae[9] or the Placodiaceae.[10][note 1] In 1971, Carroll William Dodge proposed the family Xanthoriaceae to contain Xanthodactylon, Xanthopeltis and Xanthoria,[13] but it was not validly published.[8]
In the 20th century, particularly with the widespread use of electron microscopy, the details of ascus structure became quite important considerations in the taxonomy of lichen-forming fungi.[14] In the case of the Teloschistaceae, several studies on various species noted the common presence of a strongly amyloid cap-like zone at the tip of the ascus.[15][16][17] Rosmarie Honegger, using transmission electron microscopy to better visualise structure, verified the presence of a special ascus type featuring an amyloid outer layer without visible apical structures, and with an irregular dehiscence; she named this the Teloschistes-type.[18] The presence of this ascus type was later used as a diagnostic character for the family Teloschistaceae following an ultrastructural study that corroborated her work.[19] In 1989, Ingvar Kärnefelt revised the family, accepting ten genera,[20] and this served as the main taxonomic classification for the family until the molecular era.[21] In one of the last classifications of the family prior to the widespread use and implementation of molecular techniques, Ove Eriksson, in his popular Outline of the Ascomycota series, accepted 12 genera in Teloschistaceae in 2006: Caloplaca, Cephalophysis, Fulgensia, Huea, Ioplaca, Josefpoeltia, Seirophora, Teloschistes, Xanthodactylon, Xanthomendoza, Xanthopeltis, and Xanthoria.[22] The family continues to undergo significant changes. For example, in 2020, of all fungal families, Teloschistaceae had the fourth-highest number of new fungal names (a total of 128), including 8 genera, 48 new species and infraspecific[note 2] taxa, and 72 new combinations.[23]
Etymology
As is standard practice in botanical nomenclature,[24] the name Teloschistaceae is based on the name of the type genus, Teloschistes, with the ending -aceae indicating the rank of family. The genus name, assigned by Norwegian botanist Johannes M. Norman in 1852,[25] comprises two Greek words: τέλος (télos), meaning "end", "final", or "term"; and σχιστός (-schistós), meaning "divided into", "split", or "separated".[26]
Subfamilial and ordinal classification
Teloschistaceae contains three subfamilies: Xanthorioideae, Caloplacoideae, and Teloschistoideae.[27] A fourth subfamily, Brownlielloideae, was proposed in 2015;[28] independent molecular studies, however, did not support the existence of the subfamily.[29] A later critical analysis showed it to be part of the Teloschistoideae, as it is, according to the authors, "an artifactual taxon based on a "chimeric" data set, with the type genus being part of Teloschistoideae".[30] Reanalysis of the DNA sequences representing genuine Teloschistaceae showed that the members of Brownlielloideae are dispersed within the other three subfamilies, although mostly within Teloschistoideae.[31] Similar reasoning applies to the subfamily Ikaerioideae,[32] which was informally introduced by Kondratyuk and colleagues in 2020.[33] Despite these results, Kondratyuk and colleagues continue to use these subfamilies, proposing that Brownlielloideae contains nine genera (Brownliella, Dijigiella, Lazarenkoella, Marchantiana, Raesaeneniana, Streimanniella, Tarasginia, Tayloriellina, and Thelliana) and that Ikaerioideae contains two (Ikaeria and Loekoeslaszloa).[34] Each of the three accepted subfamilies includes crustose, foliose, and fruticose forms, suggesting that shifts among these growth forms were common throughout evolutionary history.[35] These subfamilies comprise distinct clades in the phylogenetic tree of the Teloschistaceae, and are distinguished from each other by differences in the nucleic acid sequence patterns of their nuclear large ribosomal subunit.[36]
- Caloplacoideae Arup, Søchting & Frödén (2020)
- Type genus: Caloplaca. This subfamily was proposed by Ester Gaya and colleagues in 2012, used by Ulf Arup and colleagues in their 2013 publication, and finally published validly in 2020. Caloplacoideae contains mostly crustose species, and collectively has a wide distribution and a diverse secondary chemistry.[37]
- Teloschistoideae Arup, Søchting & Frödén (2020)
- Type genus: Teloschistes. This subfamily was first informally proposed (without a valid diagnosis) in 2013,[38] published the same year with a diagnosis but invalidly (without a unique identifier),[39] and finally validly published in 2020.[40] The subfamily has a largely Southern Hemisphere distribution.[39]
- Xanthorioideae Arup, Søchting & Frödén (2020)
- Type genus: Xanthoria. The name for this subfamily was originally proposed by Gaya and colleagues in 2012, and used by Arup and colleagues in 2013. The subfamily name was formally validated when it was published in 2020 with a diagnosis.[41] Most Xanthorioideae species are found in the Northern Hemisphere.[27]
The order Teloschistales was first proposed by David Hawksworth and Eriksson in 1986, with a single family (Teloschistaceae); other families were added later.[42] In the 1990s, several authors recognised the Teloschistales as a suborder within the Lecanorales;[43] as a suborder it was named Teloschistineae.[44] Following the appearance of preliminary molecular studies,[45] the Teloschistaceae was classified by some within the order Lecanorales, although others maintained the Teloschistales as a valid order.[46] The large-scale, multigene phylogenetic study of the class Lecanoromycetes by Jolanta Miądlikowska and colleagues published in 2014 corroborated the ordinal status of the Teloschistales, and showed it comprises two clades: Letrouitineae (containing Brigantiaeaceae and Letrouitiaceae) and its sister clade, Teloschistineae (containing Teloschistaceae and Megalosporaceae).[47] The suborder Teloschistineae was formally proposed by Ester Gaya and François Lutzoni in 2015.[48]
Molecular phylogenetics
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cladogram showing the phylogeny of some species and genera in family Teloschistaceae and in the order Teloschistales; based on analysis by Wilk et al. in 2021 (simplified from original).[49] Species names have been updated to reflect current taxonomy. Single quote marks around a genus name suggest that the species may be misclassified. |
In terms of the number of sequences submitted to GenBank, Teloschistaceae, with more than 6400 sequences, is second only to the Parmeliaceae.[34] As of 2023, the mycobiont genomes of about 20 Teloschistaceae species have been fully sequenced using metagenomics.[50] The proliferation of molecular techniques has increased the ability to distinguish species that were previously thought to be "phenotypically indistinguishable", as with the semi-cryptic species group containing the similarly named Caloplaca micromarina, C. micromontana, and C. microstepposa.[51]
In the previous decade, several large lichen-forming families, including the Teloschistaceae, have experienced "dramatic changes in generic concepts".[48] The earliest molecular studies involving members of the Teloschistaceae that attempted to address higher taxonomic levels usually suffered the limitations of including too few relevant example species.[52][53] Other more focused studies typically analysed only a single locus (sometimes two) of nuclear ribosomal DNA, limiting their phylogenetic resolving power. One of the first comprehensive phylogenetic studies of the Teloschistales, based on a multi-gene analysis of a large number of samples, was published by Ester Gaya and colleagues in 2012.[21]
The historically widely recognised genera within Teloschistaceae were typically distinguished based on growth form (such as Xanthoria, Caloplaca, Ioplaca, Xanthopeltis, and Xanthodactylon), the nature of the cortical layer and the existence of rhizines (as seen in Xanthomendoza, Teloschistes, and Josefpoeltia), or by the type of spore (for example, Fulgensia and Xanthopeltis). However, this method of classification was shown to have practical problems, especially when distinguishing Caloplaca from Xanthoria, as several species exhibit growth forms that fall in between. Moreover, all the cortical tissues and spore varieties present in the family can also be found within the traditionally defined Caloplaca. The widespread application of molecular techniques to the family has shown that many morphological and anatomical characters are quite variable in nature, and consequently generally unreliable as evolutionary markers.[27] Gaya and colleagues summed the taxonomic status of the family in 2012: "Our molecular phylogenetic study confirms that the genus Caloplaca was defined based on symplesiomorphic characters (such as the crustose growth form and presence of anthraquinones) rendering the taxonomic disentanglement of the Teloschistaceae virtually impossible without a broad and comprehensive molecular phylogenetic survey".[21] Around this time, Sergey Kondratyuk and colleagues, who later authored dozens of new genera in the family, emphasised the importance of using monophyletic groups to define generic groups in the Teloschistaceae, instead of the old morphology-based classification.[54]
Since the largest Teloschistaceae genus, Caloplaca, has been shown to be polyphyletic, this has led to the creation of dozens of smaller genera intended to more accurately represent phylogenetic relationships in the family. However, as Gintaras Kantvilas commented in 2016, "this new classification has not been without controversy, as well as proving unwieldy to most taxonomists working with traditional morphological and anatomical characters", and he further goes on to note that in Australia, this new classification "has not been generally taken up".[55] Robert Lücking and colleagues have noted in a 2017 review of lichen classification: "Families such as Teloschistaceae, in which a large number of changes to the genus concept have been introduced in widely dispersed studies by different authors, need to be consolidated by a broad, multi-locus analysis including all available data, and with rigorous analytical methods".[48]
The higher-level phylogenetic relationships of the Teloschistaceae and other members of the two major subclasses of Lecanoromycetes, Lecanoromycetidae and Ostropomycetidae, were clarified in a 2018 publication by Kraichak and colleagues. Teloschistaceae has a sister taxon relationship with Megalosporaceae, and the clade containing these two families is itself sister to a clade containing families Brigantiaeaceae and Letrouitiaceae.[56]
Description
_M%C3%BCll._Arg_918275.jpg.webp)
In general, Teloschistaceae members are known for their vibrant colours, spanning a spectrum of yellow, orange, and red hues, attributed to anthraquinone pigments.[1] This group of lichens demonstrates a broad range of physical forms — from the thin, encrusting (crustose) to leaf-like (foliose), or even bushy (fruticose) formations.[1][57] Although it is an atypical growth form for the Teloschistaceae, members of genus Ioplaca are somewhat umbilicate.[58]
Teloschistaceae lichens have a symbiotic relationship with a photobiont, generally a member of the green algal genus Trebouxia.[57] Their reproductive structures, or ascomata, are usually brightly coloured. These apotheciate ascomata typically have a lecanorine structure, although occasionally, biatorine or lecideine forms may manifest.[57][1] Additionally, reproductive propagules, such as isidia and soredia, can be found in select species.[1]
The ascomata encase asci, cylindrical formations that commonly contain between four to sixteen ascospores, with eight being the most prevalent count. These asci are characterised by a well-developed J+ layer amyloid cap and a rudimentary internal apical apparatus.[57] (The term "J+" refers to the positive reaction of the ascus tip to iodine, specifically when it turns blue or dark blue in the presence of iodine-based solutions like Melzer's reagent or Lugol's solution.) The translucent (hyaline) ascospores typically feature between one and three internal partitions called septa, marked by a robust central septum and a canal connecting the lumina.[57][1] Despite the polarilocular nature of ascospores suggesting Teloschistaceae lineage, these spores are often not overtly distinctive.[59] Although polarilocular ascospores were formerly considered to be a distinguishing feature of the Teloschistaceae, the addition of genera like Apatoplaca, Cephalophysis, Fulgensia, and Xanthopeltis, all of which have simple or septate spores, prompted a reevaluation of the primary characteristics defining this family.[8]
A distinctive feature of Teloschistaceae is the presence of the gelatinous paraphyses, with either unbranched or slightly branched structures culminating in bulbous ends.[1] Asexual reproduction within this family, results in the formation of pycnidial conidiomata that yield clear, either bacillar (rod-shaped) to bifusiform (double-spindle-shaped) conidia.[1][57] The intricate tissue composition of both the thallus and apothecia has been described as loosely paraplectenchymatous.[14]
|
Photobionts
In lichens, photobionts are the photosynthetic organisms that collaborate with fungal partners to enable the unique lichen symbiosis. Members of the Teloschistaceae primarily associate with trebouxioid green algal photobionts. An early study investigating the ultrastructure of the interaction between the fungus and alga in various Teloschistaceae species revealed that, in most cases, the cells were merely in close proximity to one another, with only a few instances of fungal cells invading the algal cells.[60] The widespread Xanthoria parietina species complex has been identified to be in association with various Trebouxia species, including T. arboricola, T. decolorans, and T. italiana.[61] Within the order Teloschistales, unlike the Teloschistaceae, species in the families Letrouitiaceae and Megalosporaceae primarily partner with the green algal genus Dictyochloropsis. Due to their resilience to desiccation, Trebouxia species serve as the main photobionts for lichen-forming fungi found in extreme environments such as the Antarctic, Arctic, alpine regions, and deserts, where lichens face continual exposure to intense dryness and temperature shifts.[47]
Research on Teloschistaceae photobionts has shown that all studied foliose (Xanthoria, Xanthomendoza) and fruticose (Teloschistes) types were affiliated with specificTrebouxia clades. This indicates a degree of specificity at the genus level, where only certain subclades of the Trebouxia clade are seen as suitable partners. This specificity, however, can vary based on the habitat; in extreme climates, lichens might be associated with a broader range of photobionts.[61]
Chemistry
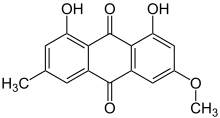
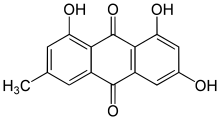
The main group of lichen products associated with the Teloschistaceae are chemical pigments called anthraquinones. These substances, which are deposited in the upper cortical layer of the lichen,[50] have photoprotective properties,[62] as they can absorb UV and blue light.[50] Evolutionary innovations in secondary metabolite production allowed the family to expand its range and switch habitat from shaded, plant-based habitats to exposed, arid habitats. The production of protective chemicals is thought to be a direct contributor to the evolutionary success of the familial lineage. A 2023 study reported using comparative genomics to identify a metabolic gene cluster involved in anthraquinone metabolism and shared uniquely across the Teloschistales. Phylogenetic analyses of fungal polyketide synthases (PKSs) reveal a consistent grouping, hinting at a shared ancestral trait for anthraquinone biosynthesis within the subphylum Pezizomycotina. While the genetic machinery (like the PKSs) involved in anthraquinone biosynthesis in Teloschistales and some non-lichenized fungi is conserved and exhibits similarities, the specific arrangement of the involved enzymes seems to be a distinguishing feature in the Teloschistales' approach to anthraquinone biosynthesis. The discovery of an ABC transporter gene within the pigment gene cluster provides clues as to how the lichens are able to accumulate large amounts of potentially toxic anthraquinone crystals in their thallus and reproductive structures.[50]
Several early chemical studies (published between the years 1897 and 1906) by Friedrich Wilhelm Zopf and Oswald Hesse involving Teloschistaceae members involved the isolation of the reddish pigment parietin from selected species.[63] Parietin is an antioxidant molecule that is produced in greater amounts (upregulated) in lichen thalli that are exposed to excess nitrogen.[64] In a 1970 publication, Johan Santesson surveyed 230 Caloplaca species for anthraquinones as part of a phytochemical study of the Teloschistaceae, and noted that the studied species could be arranged according to their anthraquinone content in thirteen "chemical groups".[63] In 1997, Ulrik Søchting analysed secondary metabolites from species of Caloplaca, Teloschistes, and Xanthoria to look for chemical patterns of consistent combinations and proportions of lichen products. He identified two chemosyndromes with parietin, emodin, teloschistin, fallacinal, and parietinic acid as the main substances.[65] Parietin acts as a UV-light filter to provide optimal light intensities for the photobionts that are resident in the internal algal layer. Investigations into the parietin concentration in Xanthoria parietina across a light gradient reveal a direct relationship between light intensity and concentration. In the Teloschistaceae, parietin may serve an additional defensive role. In the Negev desert, the parietin-containing Elenkiniana ehrenbergii and Seirophora lacunosa are avoided by snails, while they frequently consume species like Diploicia canescens and Buellia subalbula, which lack parietin.[66]
In their large-scale phylogenetic analysis of the Teloschistaceae, Arup and colleagues analysed about 4000 members of the family using high-performance liquid chromatography, and identified more than 100 secondary metabolites (lichen products), mostly anthraquinones. They noted that in the large majority of cases, the distribution of lichen products was more or less constant within species. In some instances, the secondary chemistry is important at higher taxonomic levels (i.e., a rank higher than species).[27] For example, the genus Catenaria, which contains three South American species, is characterised by the presence of 7-chlorocatenarin, a secondary metabolite previously unknown in lichens.[67] Similarly, the substance usnic acid characterises the genus Usnochroma, while 5-chloroemodin occurs in all but one species of Shackletonia. The secondary chemistry of the Caloplacoideae is the most diverse amongst the three Teloschistaceae subfamilies, as it contains both chlorinated anthraquinones and depsidones.[27]

Although most Teloschistaceae lichen produce yellow-orange-red anthraquinone pigments, Apatoplaca and Cephalophysis are two Teloschistaceae genera that lack anthraquinones. Similarly, the genus Pyrenodesmia encompasses species where anthraquinones are absent and replaced by substances such as Cinereorufa‐green or Sedifolia-grey; these pigments may confer UV-protective ability similar to anthraquinones. Taxa of the closely related genera Kuettlingeria and Sanguineodiscus have anthraquinones in their apothecia and Sedifolia-grey in their thalli.[69] The species Kuettlingeria neotaurica features apothecia of two colour variants: orange-red (with anthraquinones) and grey (with Sedifolia-grey). The absence of anthraquinones is not a synapomorphic character, but appears independently in unrelated lineages of Teloschistaceae; as such, it is a phylogenetically unreliable character.[70]
Adaptive radiation
Adaptive radiation in the Teloschistaceae has been studied to understand the key phenotypic changes leading to their diversification. This diversification is believed to be connected to the spread of anthraquinone pigments in their thallus. Initially, these pigments were thought to have appeared during the Teloschistaceae's first divergence, with a more widespread occurrence developing later. The distribution of anthraquinones varies, from being dispersed across the organism's surface to localized regions. Analysis suggests that the family's lineage witnessed a loss and subsequent return of these pigments over time, considering their presence in the thallus and apothecia as the ancestral state. Ecologically, these organisms shifted from shaded, bark-dwelling habitats to sunlit, rocky areas during their diversification.[71]
Examining the relationship between phenotypic traits and diversification rates, it becomes evident that the presence of anthraquinones in the thallus and increased sun exposure accelerated diversification. On the contrary, living in shaded environments or having a crustose-continuous (smooth, non-scaly) growth form hindered diversification. The choice of substrate, be it rock or bark, did not have a pronounced impact on diversification rates. This adaptive radiation within the Teloschistaceae is estimated to have initiated around 100 million years ago, specifically during the Late Cretaceous period. Factors like climatic shifts, continental separations, and the emergence of flowering plants are theorized to have influenced the adaptive landscape. Such factors might have promoted the development of light-protective anthraquinones, enabling Teloschistaceae to colonize exposed environments.[71] A notable aspect of their evolution is the diversification of anthraquinone genes, which is mainly attributed to gene modifications through gene reshuffling, leading to new biosynthetic enzyme pathways and gene clusters.[50]
Genera
This section presents a compilation of the genera within the Teloschistaceae, based largely on a 2021 fungal classification review.[72] Each genus is paired with its taxonomic authority, denoting the first describers using standardized author abbreviations, the publication year, and the respective number of species.
Contemporary estimates of the number of Teloschistaceae taxa include: 10 genera and 47 species (2001),[46] 12 genera and 644 species (2008);[73] 51–53 genera and about 700 species (2016);[57] 65 genera and 755 species (2017);[48] and 71 genera and about 840 species (2022).[72] Also in 2022, Kondratyuk and colleagues enumerated all members of the Teloschistaceae with available DNA sequences, and confirmed 590 species in 115 genera.[34] As of August 2023, Species Fungorum (in the Catalogue of Life), accepts 117 genera and 805 species in the Teloschistaceae. The largest genus is Caloplaca, at 173 accepted species.[74][note 3]
In terms of diversity, Teloschistaceae stood as the sixth-largest lichen-forming fungal family by 2017, following the Parmeliaceae, Graphidaceae, Verrucariaceae, Ramalinaceae, and the Lecanoraceae.[48] Genera are organised here by subfamily:
Caloplacoideae
- Apatoplaca Poelt & Hafellner (1980)[75] – 1 sp.
- Blastenia A.Massal. (1852)[3] – 11 spp.
- Bryoplaca Søchting, Frödén & Arup (2013)[76] – 3 spp.
- Caloplaca Th.Fr. (1860)[77] – 351 spp.
- Cephalophysis (Hertel) H.Kilias (1985)[78] – 1 sp.
- Eilifdahlia S.Y.Kondr., Kärnefelt, Elix, A.Thell & Hur (2014)[79] – 2 spp.
- Elenkiniana S.Y.Kondr., Kärnefelt, Elix, A.Thell & Hur (2014)[79] – 3 spp.[note 4]
- Fauriea S.Y.Kondr., Lőkös & Hur (2016)[80] – 7 spp.
- Franwilsia S.Y.Kondr., Kärnefelt, Elix, A.Thell & Hur (2014)[79] – 3 spp.
- Fulgensia A.Massal. & De Not. (1853)[81] – 2 spp.
- Gintarasiella S.Y.Kondr. & Hur (2017)[82] – 1 sp.
- Gyalolechia A.Massal. (1852)[83] – 40 spp.[note 4]
- Hanstrassia S.Y.Kondr. (2017)[84] – 2 spp.
- Huneckia S.Y.Kondr., Elix, Kärnefelt, A.Thell & Hur (2014)[79] – 4 spp.
- Ioplaca Poelt (1977)[85] – 2 spp.
- Jasonhuria S.Y.Kondr., Lőkös & S.O.Oh (2015)[86] – 1 sp.
- Klauderuiella S.Y.Kondr. & Hur (2017)[87] – 3 spp.[note 5]
- Kuettlingeria Trevis. (1857)[88] – 15 spp.
- Lacrima Bungartz, Arup & Søchting (2020)[89] – 4 spp.
- Laundonia S.Y.Kondr., Lőkös & Hur (2017)[90] – 2 spp.
- Lendemeriella S.Y.Kondr. (2020)[33] – 9 spp.
- Leproplaca (Nyl.) Nyl. (1888)[91] – 7 spp.
- Loekoesia S.Y.Kondr., S.O.Oh & Hur (2015)[86] – 1 sp.
- Marchantiana S.Y.Kondr., Kärnefelt, Elix, A.Thell & Hur (2014)[79] – 7 spp.
- Mikhtomia S.Y.Kondr., Kärnefelt, Elix, A.Thell & Hur (2014)[79] – 4 spp.[note 4]
- Obscuroplaca Søchting, Arup & Bungartz (2021)[92] – 3 spp.
- Oceanoplaca Arup, Søchting & Bungartz (2020)[93] – 6 spp.[note 6]
- Olegblumia S.Y.Kondr., Lőkös & Hur (2020)[86] – 1 sp.
- Opeltia S.Y.Kondr. & Lőkös (2017)[95] – 4 spp.
- Oxneriopsis S.Y.Kondr., Upreti & Hur (2017)[96] – 4 spp.
- Pisutiella S.Y.Kondr., Lőkös & Farkas (2020)[33] – 6 spp.
- Pyrenodesmia A.Massal. (1852)[97] – 6 spp.
- Rufoplaca Arup, Søchting & Frödén (2013)[98] – 10 spp.
- Sanguineodiscus I.V.Frolov & Vondrák (2020)[69] – 4 spp.
- Seirophora Poelt (1983)[99] – 8 spp.
- Sucioplaca Bungartz, Søchting & Arup (2020)[100] – 1 sp.
- Upretia S.Y.Kondr., A.Thell & Hur (2017)[58] – 2 spp.
- Usnochroma Søchting, Arup & Frödén (2013)[101] – 2 spp.
- Variospora Arup, Søchting & Frödén (2013)[101] – 16 spp.[note 5]
- Xanthaptychia S.Y.Kondr. & Ravera (2017)[102] – 3 spp.
- Yoshimuria S.Y.Kondr., Kärnefelt, Elix, A.Thell & Hur (2014)[79] – 4 spp.
Teloschistoideae
- Aridoplaca Wilk, Pabijan & Lücking (2021)[103] – 1 sp.
- Brownliella S.Y.Kondr., Kärnefelt, Elix, A.Thell & Hur (2013)[104] – 4 spp.
- Catenarina Søchting, Søgaard, Arup, Elvebakk & Elix (2014)[67] – 3 spp.
- Cinnabaria Wilk, Pabijan & Lücking (2021)[105] – 1 sp.
- Elixjohnia S.Y.Kondr. & Hur (2017)[106] – 4 spp.
- Filsoniana S.Y.Kondr., Kärnefelt, Elix, A.Thell & Hur (2013)[107] – 9 spp.
- Follmannia C.W.Dodge (1967)[108] – 2 spp.
- Fulgogasparrea S.Y.Kondr., M.H.Jeong, Kärnefelt, Elix, A.Thell & Hur (2013)[109] – 5 spp.[note 7]
- Haloplaca Arup, Søchting & Frödén (2013)[111] – 3 spp.
- Harusavskia S.Y.Kondr. (2017)[112] – 1 sp.
- Hosseusiella S.Y.Kondr., L.Lőkös, Kärnefelt & A.Thell (2018)[113] – 3 spp.
- Ikaeria S.Y.Kondr., D.Upreti & Hur (2017)[114] – 2 spp.
- Iqbalia Fayyaz, Afshan & S.Y.Kondr. (2022)[115] – 1 sp.
- Josefpoeltia S.Y.Kondr. & Kärnefelt (1997)[116] – 3 spp.
- Kaernefia S.Y.Kondr., Elix, A.Thell & Hur (2013)[117] – 3 spp.
- Lazarenkoiopsis S.Y.Kondr., Lőkös & Hur (2017)[118] – 1 sp.
- Loekoeslaszloa S.Y.Kondr., Kärnefelt, A.Thell & Hur (2019)[119] – 2 spp.
- Neobrownliella S.Y.Kondr., Elix, Kärnefelt & A.Thell (2015)[120] – 5 spp.
- Nevilleiella S.Y.Kondr. & Hur (2017)[121] – 2 spp.
- Niorma A.Massal. (1861)[122] – 5 spp.[note 8]
- Raesaeneniana S.Y.Kondr., Kärnefelt, A.Thell, Elix & Hur (2015)[123] – 1 sp. [note 9]
- Rehmaniella S.Y.Kondr. et al.[113] – 1 sp.
- Scutaria Søchting, Arup & Frödén (2013)[125] – 1 sp.
- Sirenophila Søchting, Arup & Frödén (2013)[27] – 4 spp.
- Stellarangia Frödén, Arup & Søchting (2013)[126] – 3 spp.
- Streimanniella S.Y.Kondr., Kärnefelt, A.Thell, Elix & Hur (2015)[127] – 4 spp.
- Tarasginia S.Y.Kondr., Kärnefelt, A.Thell, Elix & Hur (2015)[128] – 2 spp. [note 10]
- Tassiloa S.Y.Kondr., Kärnefelt, A.Thell, Elix & Hur (2015)[130] – 2 spp.
- Tayloriellina S.Y.Kondr., Kärnefelt, A.Thell, Elix & Hur (2016)[80] – 2 spp.[note 11]
- Teloschistes Norman (1852)[25] – ca. 24 spp.
- Teloschistopsis Frödén, Søchting & Arup (2013)[132] – 3 spp.
- Thelliana S.Y.Kondr., Kärnefelt, Elix & Hur (2015)[133] – 1 sp. [note 12]
- Villophora Søchting, Arup & Frödén (2013)[134] – 9 spp.
- Wetmoreana Arup, Søchting & Frödén (2013)[134] – 2 spp.
- Wilketalia S.Y.Kondr (2021)[135] – 1 sp.
Xanthorioideae
- Amundsenia Søchting, Garrido-Ben., Arup & Frödén (2014)[136] – 2 spp.
- Athallia Arup, Frödén & Søchting (2013)[137] – 17 spp.
- Austroplaca Søchting, Frödén & Arup (2013)[138] – 10 spp.
- Calogaya Arup, Frödén & Søchting (2013)[139] – 19 spp.
- Cerothallia Arup, Frödén & Søchting (2013)[140] – 4 spp.
- Charcotiana Søchting, Garrido-Ben. & Arup (2014)[136] – 1 sp.
- Coppinsiella S.Y.Kondr. & Lőkös (2018)[141] – 3 spp.
- Dijigiella S.Y.Kondr. & L.Lőkös (2017)[142] – 2 spp.[note 13]
- Dufourea Ach. (1809)[143] – 25 spp.[note 14]
- Erichansenia S.Y.Kondr., Kärnefelt & A.Thell (2020)[33] – 3 spp.
- Flavoplaca Arup, Søchting & Frödén (2013)[144] – 28 spp.
- Fominiella S.Y.Kondr., Upreti & Hur (2017)[145] – 2 spp.
- Gallowayella S.Y.Kondr., Fedorenko, S.Stenroos, Kärnefelt, Elix & A.Thell (2012)[146] – 15 spp.[note 15]
- Golubkovia S.Y.Kondr., Kärnefelt, Elix, A.Thell & Hur (2014)[147] – 1 sp.[note 15]
- Gondwania Søchting, Frödén & Arup (2013)[148] – 4 spp.
- Honeggeria S.Y.Kondr., Fedorenko, S.Stenroos, Kärnefelt, Elix, Hur & A.Thell (2012)[146] – 1 sp.[note 15]
- Huriella S.Y.Kondr. (2017)[149] – 5 spp.[note 16]
- Igneoplaca S.Y.Kondr., Kärnefelt, Elix, A.Thell & Hur (2014)[147] – 1 sp.
- Jackelixia S.Y.Kondr., Fedorenko, S.Stenroos, Kärnefelt & A.Thell (2009)[147][note 14]
- Jesmurraya S.Y.Kondr., Fedorenko, S.Stenroos, Kärnefelt, Elix, Hur & A.Thell (2012)[146] – 1 sp.[note 15]
- Kudratoviella S.Y.Kondr., L.Lőkös, Kärnefelt & A.Thell (2022)[94] – 5 spp.
- Langeottia S.Y.Kondr., Kärnefelt, Elix, A.Thell & Hur (2014)[147] – 2 spp.[note 14]
- Lazarenkoella S.Y.Kondr., Kärnefelt, A.Thell, Elix & Hur (2015)[151] – 2 spp.
- Martinjahnsia S.Y.Kondr., Fedorenko, S.Stenroos, Kärnefelt, Elix, Hur & A.Thell (2012)[146] – 1 sp.
- Massjukiella S.Y.Kondr., Fedorenko, S.Stenroos, Kärnefelt, Elix, Hur & A.Thell (2012)[146] – 8 spp.
- Orientophila Arup, Søchting & Frödén (2013)[152] – 4 spp.
- Ovealmbornia S.Y.Kondr., Fedorenko, S.Stenroos, Kärnefelt, Elix & A.Thell (2009)[153] – 3 spp.[note 14]
- Oxneria S.Y.Kondr. & Kärnefelt (2003)[154] – 4 spp.[note 15]
- Pachypeltis Søchting, Arup & Frödén (2013)[155] – 7 spp.
- Parvoplaca Arup, Søchting & Frödén (2013)[156] – 6 spp.
- Pisutiella S.Y.Kondr., Lőkös & Farkas (2020)[33] – 5 spp.
- Polycauliona Hue (1908)[157] – 18 spp.
- Rusavskia S.Y.Kondr. & Kärnefelt (2003)[154] – 19 spp.
- Scythioria S.Y.Kondr., Kärnefelt, Elix, A.Thell & Hur (2014)[147] – 3 spp.
- Seawardiella S.Y.Kondr., I.Kärnefelt & A.Thell (2018)[141] – 2 spp.
- Shackletonia Søchting, Frödén & Arup (2013)[158] – 5 spp.
- Solitaria Arup, Søchting & Frödén (2013)[158] – 1 sp.
- Squamulea Arup, Søchting & Frödén (2013)[158] – 15 spp.
- Teuvoahtiana S.Y.Kondr. & Hur (2017)[159] – 3 spp.
- Tomnashia S.Y.Kondr. & Hur (2017)[160] – 4 spp.
- Verrucoplaca S.Y.Kondr., Kärnefelt, Elix, A.Thell & Hur (2014)[147] – 1 sp.
- Xanthocarpia A.Massal. & De Not. (1853)[81] – 12 spp.
- Xanthodactylon P.A.Duvign. (1941)[161] – 2 spp.
- Xanthokarrooa S.Y.Kondr., Fedorenko, S.Stenroos, Kärnefelt, Elix & A.Thell (2009)[153] – 2 spp.[note 14]
- Xanthomendoza S.Y.Kondr. & Kärnefelt (1997)[116] – 20 spp.[note 15]
- Xanthopeltis R.Sant. (1949)[162] – 1 sp.
- Xanthoria (Fr.) Th.Fr. (1860)[77] – 10 spp.
- Zeroviella S.Y.Kondr. & Hur (2015)[163] – 8 spp.
Some of the genera proposed during the recent restructuring of the family have since been shown to be nomenclaturally illegitimate or unavailable for use. For example,
- Andina Wilk, Pabijan & Lücking (2021) has been replaced with Wilketalia.[135]
- Phaeoplaca Søchting, Arup & Bungartz (2020) has been replaced with Obscuroplaca.[92]
- Tayloriella S.Y.Kondr., Kärnefelt, A.Thell, Elix & Hur (2015) has been replaced with Tayloriellina.[80]
Habitat, distribution, and ecology
Collectively, the family has a cosmopolitan distribution, although members occur predominantly in subtropical and temperate regions. Most members either grow on rock (saxicolous) or on bark (corticolous).[57] As an exception to this general ecological preference, genus Bryoplaca, contains species that only grow on mosses and detritus.[27] Some Fulgensia species grow on soil (terricolous), particularly those rich in lime.[164] Several crustose Teloschistaceae species, typically saxicolous in nature, have been recorded growing on human bone remains recovered at a looted Late Holocene aboriginal cairn burial site in South America.[165]
In general, the family is moderately to strongly nitrophilous. This suggests a preference of many of its species for habitats that are rich in nitrogen, particularly in the form of nitrate.[14] Sun-adapted lichens, such as the Teloschistaceae, have an enhanced ability to up-regulate carbon fixation and absorb excess nitrogen. Further, small foliose and crustose lichens are in general more tolerant to nitrogen.[64] Xanthoria parietina is one example of a widespread lichen that appears to be experiencing an increase in its range due to its ability to tolerate nitrogenous pollutants, and a potential ability to displace native lichen species as a result.[166] Caloplaca, Fulgensia, Teloschistes, and Xanthoria are genera that are characteristic of exposed habitats; in some extreme desert environments, Caloplaca (in the broad sense) may be the only genus present, while Caloplaca and Xanthoria dominate harsh coastal environments.[66]
There are several Teloschistaceae genera that contain lichenicolous (lichen-dwelling) species. These originate from subfamily Caloplacoideae: Caloplaca (26 spp.), Gyalolechia (1 sp.), Variospora (1 sp.); from subfamily Teloschistoideae: Catenarina (1 sp.), Sirenophila 1; and from subfamily Xanthorioideae: Flavoplaca (4 spp.), Pachypeltis (1 sp.), and Shackletonia (3 spp.).[167] Lichenicolous species within the Teloschistaceae generally exhibit a broad range of hosts. Their distribution seems to be influenced not just by the classification of their host lichen, but also by the substrate they grow on.[168]
Teloschistaceae has a high diversity in polar regions and a substantial number of bipolar species, i.e., species occurring in both northern and southern hemispheres but largely absent from intermediate, tropical latitudes.[169] Examples include Xanthomendoza borealis, Austroplaca soropelta, and Caloplaca phlogina. Conversely, there is a relatively low diversity of crustose Teloschistaceae in Central Europe. Localised exceptions occur in primarily in sunlit locations with either calcareous or nutrient-rich siliceous formations; these habitats are predominant in the alpine regions of the Alps and the Carpathian Mountains, as well as in the arid, warm rocky steppes.[171] Some Teloschistaceae genera have a strong geographic centre of species richness; examples include Elixjohnia (Australasia),[106] Orientophila (east Asia), Shackletonia (Antarctic and subantarctic), Stellarangia (south-western Africa) and Xanthoria (Mediterranean area).[27]
Several studies published in the previous decade have enumerated the Teloschistaceae taxa occurring in certain defined geographical areas. These include:
- Altai-Sayan region, 103 species in 31 genera[172]
- Crimea, 119 species[173]
- Dagestan, 85 species[173]
- India, 115 species in 36 genera [174]
- Italy, about 160 species[4]
- Galápagos Islands, 24 species[175]
- Mexico, 142 species in 6 genera[176]
- New Zealand, about 100 species[177]
- Russian Far East, 84 species[173]
- Ural, 81 species[173]
Human interactions and uses
Economic significance

There are no species in the Teloschistaceae that have any major economic significance.[1] The ability of some members to grow on rock surfaces, however, has led to several recorded instances where Teloschistaceae species have damaged marble surfaces. In some cases, the lichens, the major contributor of which was Xanthocarpia feracissima, penetrated up to 10 mm (3⁄8 in) into the stone along larger cracks and 0.05 mm (1⁄500 in) beneath loose surface crystals, leading to crumbling of the marble surface.[178] Caloplaca pseudopoliotera and C. cupulifera are two crustose species implicated in the slow degradation of the Konark Sun Temple in India.[179]
Traditional medicine
Several Teloschistaceae species have been used in traditional medicines. Polycauliona candelaria was used in Europe (early modern era) where it was boiled with milk to treat jaundice, along with Xanthoria parietina. Rusavskia elegans was used in Afghanistan, where it was ground and applied to infected wounds for humans and livestock (Wakhan region). In Kyrgyzstan, it was ground and applied to wounds on livestock; or ground, mixed in butter, and fed to livestock (especially yak calves) with diarrhea. Teloschistes flavicans is used in China to "clear heat in the lung and liver", and to remove toxins. Xanthomendoza fallax is used in Traditional Tibetan medicine.[180]
The common and widespread species Xanthoria parietina has a long history of traditional use. It has been recorded for several uses in various regions of Spain, including: as a decoction in wine for menstrual complaints; as a decoction in water for kidney disorders; as a decoction in water for toothaches; as an analgesic for several pains; and as an ingredient in a cough syrup. In early modern era Europe, it was boiled with milk to treat jaundice (along with Polycauliona candelaria), used for diarrhea and dysentery, to stop bleeding, as a quinine replacement for malaria, and for hepatitis. In Traditional Chinese medicine the lichen has been used as an antibacterial.[180]
In science
Xanthoria parietina has been suggested for use as a potential reliable pollutant tolerance biomonitor in urban ecosystems due to its widespread presence and ability to adapt to high pollution levels.[181] Rusavskia elegans has been studied in experiments where specimens were exposed to outer space conditions, including extreme temperatures, ultraviolet radiation, and ultra-high vacuum. The results showed the lichen to have an impressive ability to survive under these conditions.[182]
Conservation

Three Teloschistaceae species have been assessed for the global IUCN Red List: Caloplaca rinodinae-albae (vulnerable, 2017),[183] Seirophora aurantiaca (endangered, 2020),[184] and Teloschistes peruensis (critically endangered, 2021).[185] Other Teloschistaceae members, some with limited geographic distributions, make appearances on regional lists. For example, the crustose New Zealand endemic Caloplaca allanii, first documented in 1932, was not collected again until 81 years later; because of its sparsity and small total area of occupancy, it has been assessed as "Threatened/Nationally Critical" using the New Zealand Threat Classification System.[186]
In some large geographical areas, the full extent of the diversity of Teloschistaceae taxa is not well known. Examples include South America, where the family has not historically received much attention,[187] and China, where of 2,164 lichen species evaluated for inclusion on its red list, only 49 were members of the Teloschistaceae; 13 of those were listed as least-concern species, and the other 36 as data deficient.[188]
Notes
- Neither Blasteniaceae C.W.Dodge & G.E.Baker (1938) nor Placodiaceae A.Fisch. (1871) are synonyms of Teloschistaceae, because neither of these families were published validly.[11][12]
- The term "infraspecific" refers to a taxonomic rank below that of species, including subspecies, variety, and form.
- Species Fungorum's recognition of Teloschistaceae species is based on their taxonomic evaluation, possibly not encapsulating the entirety of the family's species diversity.
- Kondratyuk and colleagues suggested that Gyalolechia was polyphyletic, and split it into Elenkiniana, Gyalolechia, and Mikhtomia; Wilk and colleagues maintain Arup et al.'s 2013 classification pending further research.[32]
- Kondratyuk and colleagues suggested that Variospora was polyphyletic, and split it into Klauderuiella and Variospora; Wilk and colleagues maintain Arup et al.'s 2013 classification pending further research.[32]
- Kondratyuk and colleagues suggest that Oceanoplaca is a synonym of Loekoeslaszloa.[94]
- Fulgogasparrea was proposed by Kondratyuk et al. in 2013 to resolve putative polyphyly in genus Wetmoreana; Wilk and colleagues instead prefer to use a more broadly defined genus Wetmoreana pending further studies.[110]
- Niorma was proposed by Kondratyuk et al. in 2013 for the species group centred around Teloschistes hypoglaucus; Wilk and colleagues instead use the more inclusive Teloschistes as per Arup et al. (2013) pending further studies.[110]
- Wilk and colleagues suggest that Raesaeneniana is a putative synonym of Villophora, a genus with which its single species fits well morphologically.[124]
- Wilk and colleagues proposed to reduce Tarasginia to synonymy with Sirenophila.[129]
- Wilk and colleagues proposed to reduce Tayloriellina to synonymy with Villophora.[131]
- Wilk and colleagues suggest that Thelliana is a putative synonym of Filsoniana.[124]
- Wilk and colleagues suggest that Dijigiella is a putative synonym of Teuvoahtiana.[124]
- Kondratyuk and colleagues in 2014 suggested that the genus Dufourea was polyphyletic, and divided it into four genera: Dufourea, Jackelixia, Langeottia, Ovealmbornia, and Xanthokarooa; Wilk and colleagues prefer to retain a more broadly defined Dufourea pending additional research.[110]
- Kondratyuk and colleagues suggested that Xanthomendoza was polyphyletic, and split it into Gallowayella, Golubkovia, Oxneria, Honeggeria, Jesmurraya, and Xanthomendoza; Wilk and colleagues agree that Xanthomendoza is polyphyletic, but prefer a conservative approach (maintaining Arup et al.'s 2013 classification) pending further research.[110]
- Bungartz and colleagues synonymise Huriella with Squamulea.[150]
Citations
- Cannon, Paul F.; Kirk, Paul M. (2007). Fungal Families of the World. Wallingford: CAB International. pp. 345–346, 440. ISBN 978-0-85199-827-5. OCLC 60741230.
- Jørgensen, Per M. (1994). "Linnaean lichen names and their typification". Botanical Journal of the Linnean Society. 115 (4): 261–405. doi:10.1111/j.1095-8339.1994.tb01784.x.
- Massalongo, A.B. (1852). "Synopsis Lichenum Blasteniospororum". Flora or Botanical Newspaper: Which Contains Reviews, Treatises, Essays, News and News Concerning Botany (in Latin). 35: 573.
- Nimis, Pier Luigi (2016). The Lichens of Italy. A Second Annotated Catalogue. Trieste: Edizioni Università di Trieste. pp. 7, 24. ISBN 978-88-8303-755-9.
- Zahlbruckner, A. (1898). "Flechten (Lichenes). B. Spezieller Teil". In Engler, Adolf (ed.). Syllabus der Pflanzenfamilien 2 [Syllabus of Plant Families 2.] (in German). Berlin: Gebrüder Borntraeger Verlagsbuchhandlung. p. 45.
- Döring, Heidi; Lumbsch, H. Thorsten (1998). "Ascoma ontogeny: is this character set of any use in the systematics of lichenized ascomycetes?". The Lichenologist. 30 (4–5): 489–500. doi:10.1006/lich.1998.0144. S2CID 86114296.
- Zahlbruckner, A. (1926). "Lichenes (Flechten). B. Spezieller Teil". In Engler, A.; Prantl, K. (eds.). Die natürlichen Pflanzenfamilien 8, Aufl. 2 [Lichens. B. Special Part]. Leipzig: Engelmann. pp. 61–270.
- Gaya, Ester; Navarro-Rosinés, Pere; Llimona, Xavier; Hladun, Néstor; Lutzoni, François (2008). "Phylogenetic reassessment of the Teloschistaceae (lichen-forming Ascomycota, Lecanoromycetes)". Mycological Research. 112 (5): 528–546. doi:10.1016/j.mycres.2007.11.005. PMID 18406120.
- Dodge, C.W.; Baker, G.E. (1938). "The Second Byrd Antarctic Expedition-Botany. II. Lichens and Lichen parasites". Annals of the Missouri Botanical Garden. 25 (2): 515–727. doi:10.2307/2394232. JSTOR 2394232.
- Räsänen, V. (1943). "Das System der Flechten" [The system of lichens]. Acta Botanica Fennica (in German). 33: 1–82.
- "Record Details: Blasteniaceae C.W. Dodge & G.E. Baker, Ann. Mo. bot. Gdn 25(2): 604 (1938)". Index Fungorum. Retrieved 23 August 2023.
- "Record Details: Placodiaceae A. Fisch. [as 'Placodinae'], Mitt. berin. naturf. Ges.: 17 (1871)". Index Fungorum. Retrieved 23 August 2023.
- Dodge, C. (1971). Some lichens of tropical Africa. V. Lecanoraceae to Physciaceae. Beihefte zur Nova Hedwigia. Vol. 38. p. 115. ISBN 978-3-7682-5438-0.
- Ahmadjian, Vernon; Hale, Mason E. (1973). The Lichens. New York: Academic Press. p. 625. ISBN 978-0-12-044950-7.
- Richardson, D.H.S (1970). "Ascus and ascocarp structure in lichens". The Lichenologist. 4 (4): 350–361. doi:10.1017/s0024282970000440. S2CID 85251464.
- Letrouit-Galinou, Marie-Agnès (1973). "Les asques des lichens et le type archaeascé" [The ascus of lichens and the archaeascid type]. The Bryologist (in French). 76 (1): 30–47. doi:10.2307/3241230. JSTOR 3241230.
- Henssen, Aino; Jahns, Hans Martin (1974). Lichenes. Eine Einführung in die Flechtenkunde [Lichens: An Introduction to Lichenology] (in German). Stuttgart: George Thieme Verlag. ISBN 978-3-13-496601-5.
- Honegger, R. (1978). "The ascus apex in lichenized fungi I. The Lecanora-, Peltigera- and Teloschistes-types" (PDF). The Lichenologist. 10 (1): 47–67. doi:10.1017/s0024282978000079. S2CID 84629945.
- Bellemère, A.; Hafellner, J.; Letrouit-Galinou, M.-A. (1986). "Ultrastructure et mode de déhiscence des asques chez les lichens des genres Teloschistes et Apatoplaca (Teloschistaceae)" [Ultrastructure and dehiscence mode of asci in lichens of the genera Teloschistes and Apatoplaca (Teloschistaceae)]. Cryptogamie. Bryologie, Lichenologie (in French). 7 (3): 189–211.
- Kärnefelt, I. (1989). "Morphology and phylogeny in the Teloschistales". Cryptogamic Botany. 1: 147–203.
- Gaya, Ester; Högnabba, Filip; Holguin, Ángela; Molnar, Katalin; Fernández-Brime, Samantha; Stenroos, Soili; Arup, Ulf; Søchting, Ulrik; Boom, Pieter Van den; Lücking, Robert; Sipman, Harrie J.M.; Lutzoni, François (2012). "Implementing a cumulative supermatrix approach for a comprehensive phylogenetic study of the Teloschistales (Pezizomycotina, Ascomycota)". Molecular Phylogenetics and Evolution. 63 (2): 374–387. doi:10.1016/j.ympev.2012.01.012. PMID 22306043.
- Eriksson, O.E. (2006). "Outline of Ascomycota – 2006". Myconet. 12: 1–82.
- Wang, Ke; Cai, Lei; Yao, Yijian (2021). "Overview of nomenclature novelties of fungi in the world and China (2020)". Biodiversity Science. 29 (8): 1064–1072. doi:10.17520/biods.2021202. S2CID 240568551.
- Hawksworth, D.L. (1974). Mycologist's Handbook. Kew: Commonwealth Mycological Institute. p. 39. ISBN 978-0-85198-300-4.
- Norman, J.M. (1852). "Conatus praemissus redactionis novae generum nonnullorum Lichenum in organis fructificationes vel sporis fundatae" [The preliminary effort of the new revision of certain genera of lichens based on the fructifications or spores within their structures]. Nytt Magazin for Naturvidenskapene (in Latin). 7: 213–252 [228].
- Ulloa, Miguel; Aguirre-Acosta, Elvira (2020). Illustrated Generic Names of Fungi. APS press. pp. 362–363. ISBN 978-0-89054-618-5.
- Arup, Søchting & Frödén 2013.
- Kondratyuk et al. 2015b.
- Vondrák, Jan; Shahidin, Hurnisa; Moniri, Mahroo Haji; Halıcı, Gökhan; Košnar, Jiří (2018). "Taxonomic and functional diversity in Calogaya (lichenised Ascomycota) in dry continental Asia". Mycological Progress. 17 (8): 897–916. doi:10.1007/s11557-018-1402-9. S2CID 255312095.
- Wilk et al. 2021, p. 278.
- Wilk et al. 2021, p. 283.
- Wilk et al. 2021, p. 285.
- Kondratyuk, S.Y.; Lőkös, L.; Farkas, E.; Kärnefelt, I.; Thell, A.; Yamamoto, Y.; Hur, J.-S. (2020). "Three new genera of the Teloschistaceae proved by three gene phylogeny" (PDF). Acta Botanica Hungarica. 62 (1–2): 109–136. doi:10.1556/034.62.2020.1-2.7. S2CID 226056287.
- Kondratyuk, S.Y.; Popova, L.P.; Kondratiuk, A.S.; Lőkös, L. (2022). "The first enumeration of members of the Teloschistaceae (lichen-forming Ascomycetes) status of which confirmed by three gene phylogeny" (PDF). Studia botanica hungarica. 53 (2): 137–234. doi:10.17110/studbot.2022.53.2.137. S2CID 256569290.
- Arup, Søchting & Frödén 2013, pp. 285–287.
- Bungartz, Søchting & Arup 2020, pp. 528, 563.
- Bungartz, Søchting & Arup 2020, p. 528.
- Arup, Søchting & Frödén 2013, p. 60.
- Arup, Ulf; Søchting, Ulrik; Frödén, Patrik (2013b). "Addendum to 'A new taxonomy of the family Teloschistaceae'". Nordic Journal of Botany. 31 (2): 256. doi:10.1111/j.1756-1051.2013.00295.x.
- Bungartz, Søchting & Arup 2020, p. 552.
- Bungartz, Søchting & Arup 2020, p. 563.
- Hawksworth, D.L.; Eriksson, O.E. (1986). "The names of accepted orders of ascomycetes". Systema Ascomycetum. 5 (2): 175–184.
- Hafellner, J.; Hertel, H.; Rambold, G.; Timdal, E. (1994). "Discussion 4: Lecanorales". In Hawksworth, D.L. (ed.). Ascomycete Systematics: problems and perspectives in the nineties. NATO Advanced Science Institutes Series A269. New York: Plenum Press. pp. 379–387. ISBN 978-0-306-44882-9.
- Tehler, A.; Wedin, Mats (2008). "Systematics of lichenized fungi". In Nash III, Thomas H. (ed.). Lichen Biology (2nd ed.). Cambridge, UK: Cambridge University Press. p. 349. ISBN 978-0-521-69216-8.
- Grube, Martin; Winka, Katarina (2002). "Progress in understanding the evolution and classification of lichenized ascomycetes". Mycologist. 16 (2): 67–76. doi:10.1017/S0269-915X(02)00206-9 (inactive 2023-08-23).
{{cite journal}}: CS1 maint: DOI inactive as of August 2023 (link) - Kirk, P.M.; Cannon, P.F.; David, J.C.; Stalpers, J.A. (2001). "Teloschistaceae". Ainsworth & Bisby's Dictionary of the Fungi (9th ed.). Oxon, UK: CABI Bioscience. pp. 515–516. ISBN 978-0-85199-377-5.
- Miadlikowska, Jolanta; Kauff, Frank; Högnabba, Filip; Oliver, Jeffrey C.; Molnár, Katalin; Fraker, Emily; et al. (2014). "A multigene phylogenetic synthesis for the class Lecanoromycetes (Ascomycota): 1307 fungi representing 1139 infrageneric taxa, 317 genera and 66 families". Molecular Phylogenetics and Evolution. 79: 132–168. doi:10.1016/j.ympev.2014.04.003. PMC 4185256. PMID 24747130.
- Lücking, Robert; Hodkinson, Brendan P.; Leavitt, Steven D. (2017). "The 2016 classification of lichenized fungi in the Ascomycota and Basidiomycota–Approaching one thousand genera". The Bryologist. 119 (4): 361–416. doi:10.1639/0007-2745-119.4.361. S2CID 90258634.
- Wilk et al. 2021, p. 282.
- Llewellyn, Theo; Nowell, Reuben W.; Aptroot, Andre; Temina, Marina; Prescott, Thomas A.K.; Barraclough, Timothy G.; Gaya, Ester (2023). "Metagenomics shines light on the evolution of "sunscreen" pigment metabolism in the Teloschistales (lichen-forming Ascomycota)". Genome Biology and Evolution. 15 (2). doi:10.1093/gbe/evad002. PMC 9907504. PMID 36634008.
- Frolov, Ivan; Vondrák, Jan; Fernández-Mendoza, Fernando; Wilk, Karina; Khodosovtsev, Alexander; Halıcı, Mehmet Gökhan (2016). "Three new, seemingly-cryptic species in the lichen genus Caloplaca (Teloschistaceae) distinguished in two-phase phenotype evaluation". Annales Botanici Fennici. 53 (3–4): 243–262. doi:10.5735/085.053.0413. hdl:10995/117780. S2CID 88825723.
- Lumbsch, H.Thorsten; Schmitt, Imke; Palice, Zdenek; Wiklund, Elisabeth; Ekman, Stefan; Wedin, Mats (2004). "Supraordinal phylogenetic relationships of Lecanoromycetes based on a Bayesian analysis of combined nuclear and mitochondrial sequences". Molecular Phylogenetics and Evolution. 31 (3): 822–832. doi:10.1016/j.ympev.2003.11.001. PMID 15120381.
- Peršoh, Derek; Beck, Andreas; Rambold, Gerhard (2004). "The distribution of ascus types and photobiontal selection in Lecanoromycetes (Ascomycota) against the background of a revised SSU nrDNA phylogeny". Mycological Progress. 3 (2): 103–121. doi:10.1007/s11557-006-0081-0. S2CID 495074.
- Kondratyuk, S.Y.; Jeong, M.-H.; Kärnefelt, I.; Hur, J.-S.; Thell, Arne (2012). "ФІЛОГЕНІЯ І ТАКСОНОМІЯ РОДИНИ TELOSCHISTACEAE (ASCOMYCOTA): ВАЖЛИВІСТЬ МОНОФІЛЕТИЧНИХ ГРУП" [Phylogeny and taxonomy of the Teloschistaceae (Ascomycota): Importance of monophyletic groups] (PDF). Modern Phytomorphology (in Ukrainian). 1: 53–57.
- Kastvilas, Gintaras (2016). "A synopsis and key for the lichen genus Caloplaca (Teloschistaceae) on Kangaroo Island, with the description of two new species". Journal of the Adelaide Botanic Gardens. 29: 53–69.
- Kraichak, Ekaphan; Huang, Jen-Pan; Nelsen, Matthew; Leavitt, Steven D.; Lumbsch, H. Thorsten (2018). "A revised classification of orders and families in the two major subclasses of Lecanoromycetes (Ascomycota) based on a temporal approach". Botanical Journal of the Linnean Society. 188 (3): 233–249. doi:10.1093/botlinnean/boy060. S2CID 92280920.
- Jaklitsch, Walter; Baral, Hans-Otto; Lücking, Robert; Lumbsch, H. Thorsten (2016). Frey, Wolfgang (ed.). Syllabus of Plant Families: Adolf Engler's Syllabus der Pflanzenfamilien. Vol. 1/2 (13 ed.). Berlin Stuttgart: Gebr. Borntraeger Verlagsbuchhandlung, Borntraeger Science Publishers. pp. 136–137. ISBN 978-3-443-01089-8. OCLC 429208213.
- Kondratyuk, S.Y.; Persson, P.E.; Hansson, M.; Mishra, G.K.; Nayaka, S.; Liu, D.; Hur, J.S.; Thell, A. (2018). "Upretia, a new caloplacoid lichen genus (Teloschistaceae, lichen-forming Ascomycota) from India". Cryptogam Biodiversity and Assessment. Special volume (2018): 22–31. doi:10.21756/CAB.ESP5. S2CID 89792633.
- Hafellner, Josef (1988). "Principles of classification and main taxonomic groups". In Galun, Margalith (ed.). CRC Handbook of Lichenology. Vol. III. Boca Raton: CRC Press. p. 42. ISBN 978-0-8493-3583-9.
- Galun, Margalith; Ben-Shaul, Y.; Paran, Navah (1971). "Fungus-alga association in lichens of the Teloschistaceae: an ultrastructural study". New Phytologist. 70 (5): 837–839. doi:10.1111/j.1469-8137.1971.tb02584.x.
- Nyati, Shyam; Scherrer, Sandra; Werth, Silke; Honegger, Rosmarie (2014). "Green-algal photobiont diversity (Trebouxia spp.) in representatives of Teloschistaceae (Lecanoromycetes, lichen-forming ascomycetes)" (PDF). The Lichenologist. 46 (2): 189–212. doi:10.1017/s0024282913000819. S2CID 46909050.
- Nguyen, Khanh-Hung; Chollet-Krugler, Marylène; Gouault, Nicolas; Tomasi, Sophie (2013). "UV-protectant metabolites from lichens and their symbiotic partners". Natural Product Reports. 30 (12): 1490–1508. doi:10.1039/c3np70064j. PMID 24170172.
- Santesson, Johan (1970). "Anthraquinones in Caloplaca". Phytochemistry. 9 (10): 2149–2166. Bibcode:1970PChem...9.2149S. doi:10.1016/s0031-9422(00)85380-7.
- Delves, Jay; Lewis, Jason E.J.; Ali, Niaz; Asad, Saeed A.; Chatterjee, Sudipto; Crittenden, Peter D.; et al. (1 July 2023). "Lichens as spatially transferable bioindicators for monitoring nitrogen pollution". Environmental Pollution. 328: 121575. doi:10.1016/j.envpol.2023.121575. PMID 37028790.
- Søchting, Ulrik (1997). "Two major anthraquinone chemosyndromes in Teloschistaceae". In Türk, Roman; Zorer, Roberto (eds.). Progress and Problems in Lichenology in the Nineties – IAL 3. Bibliotheca Lichenologica. Vol. 68. Berlin/Stuttgart: J. Cramer. pp. 135–144. ISBN 978-3-443-58047-6.
- Rundel, Philip (1978). "The ecological role of secondary lichen substances". Biochemical Systematics and Ecology. 6 (3): 157–170. doi:10.1016/0305-1978(78)90002-9.
- Søchting, Ulrik; Søgaard, Majbrit Zeuthen; Elix, John A.; ARUP, Ulf; Elvebakk, Arve; Sancho, Leopoldo G. (2014). "Catenarina (Teloschistaceae, Ascomycota), a new Southern Hemisphere genus with 7-chlorocatenarin". The Lichenologist. 46 (2): 175–187. doi:10.1017/s002428291300087x. S2CID 83906534.
- Sipman, Harrie; Aptroot, André (2020). "Ikaeria serusiauxii, a new Caloplaca-like lichen from Macaronesia and mainland Portugal, with a lichen checklist for Porto Santo". Plant and Fungal Systematics. 65: 120–130. doi:10.35535/pfsyst-2020-0006.
- Frolov, Ivan; Vondrák, Jan; Košnar, Jiří; Arup, Ulf (2021). "Phylogenetic relationships within Pyrenodesmia sensu lato and the role of pigments in its taxonomic interpretation". Journal of Systematics and Evolution. 59 (3): 454–474. doi:10.1111/jse.12717. S2CID 234535735.
- Vondrák, Jan; Šoun, Jaroslav; Vondrákov, Olga; Fryday, Alan M.; Khodosovtsev, Alexander; Davydov, Evgeny A. (2012). "Absence of anthraquinone pigments is paraphyletic and a phylogenetically unreliable character in the Teloschistaceae". The Lichenologist. 44 (3): 401–418. doi:10.1017/s0024282911000843. S2CID 54693944.
- Gaya, Ester; Fernández-Brime, Samantha; Vargas, Reinaldo; Lachlan, Robert F.; Gueidan, Cécile; Ramírez-Mejía, Martín; Lutzoni, François (2015). "The adaptive radiation of lichen-forming Teloschistaceae is associated with sunscreening pigments and a bark-to-rock substrate shift". Proceedings of the National Academy of Sciences. 112 (37): 11600–11605. Bibcode:2015PNAS..11211600G. doi:10.1073/pnas.1507072112. PMC 4577145. PMID 26324894.
- Wijayawardene, N.N.; Hyde, K.D.; Dai, D.Q.; Sánchez-García, M.; Goto, B.T.; Saxena, R.K.; et al. (2022). "Outline of Fungi and fungus-like taxa – 2021". Mycosphere. 13 (1): 53–453 [156–158]. doi:10.5943/mycosphere/13/1/2. S2CID 249054641.
- Kirk, P.M.; Cannon, P.F.; Minter, D.W.; Stalpers, J.A., eds. (2008). "Teloschistaceae". Dictionary of the Fungi (10th ed.). Wallingford, UK: CAB Europe. p. 680. ISBN 978-0-85199-826-8.
- Source dataset. Species Fungorum Plus: Species Fungorum for CoL+. "Teloschistaceae". Catalogue of Life Version: 2023-07-18. Retrieved 23 August 2023.
- Poelt, J.; Hafellner, J. (1980). "Apatoplaca - Genus novum Teloschistacearum (Lichenes)" [Apatoplaca – A new genus of Teloschistaceae (lichens)]. Mitteilungen aus der Botanischen Staatssammlung München (in German). 16: 503–528.
- Arup, Søchting & Frödén 2013, p. 68.
- Fries, Theodore Magnus (1860). Lichenes Arctoi Europae Groenlandiaeque hactenus cogniti [Lichens of Arctic Europe and Greenland hitherto known] (in Latin). Uppsala: Leffler. pp. 166, 218.
- Kilias, H. (1985). "Cephalophysis (Hertel) Kilias gen. nov., eine weitere Gattung der Teloschistaceae mit einzelligen Sporen" [Cephalophysis (Hertel) Kilias gen. nov., another genus of the Teloschistaceae family with single-celled spores]. Herzogia. 7 (1–2): 181–190. doi:10.1127/herzogia/7/1985/181. S2CID 249722636.
- Kondratyuk, S.Y.; Jeong, M.-H.; Yu, N.-N.; Kärnefelt, I.; Thell, A.; Elix, J.A.; Kim, J.; Kondratiuk, A.S.; Hur, J.-S. (2014). "A revised taxonomy for the subfamily Caloplacoideae (Teloschistaceae, Ascomycota) based on molecular phylogeny". Acta Botanica Hungarica. 56 (1–2): 93–123. doi:10.1556/abot.56.2014.1-2.10.
- Kondratyuk, S.Y.; Lőkös, L.; Kim, J.A.; Kondratiuk, A.S.; Jeong, M.-H.; Jang, S.H.; Oh, S.-O.; Wang, X.Y.; Hur, J.-S. (2016). "Fauriea, a new genus of the lecanoroid caloplacoid lichens (Teloschistaceae, lichen-forming ascomycetes)" (PDF). Acta Botanica Hungarica. 58 (3–4): 303–318. doi:10.1556/abot.58.2016.3-4.6.
- Massalongo, A. (1853). Alcuni generi di licheni nuovamente limitati e descritti [Some genera of lichens newly delimited and described] (in Latin). Verona: Antonelli. pp. 10–11.
- Kondratyuk et al. 2017, p. 90.
- Massalongo, A.B. (1852). Ricerche sull'autonomia dei licheni crostosi [Research on the autonomy of crustose lichens]. Verona: Dalla tipografia di A. Frizierio. p. 17.
- Kondratyuk et al. 2017, p. 91.
- Poelt, J. (1977). "Ioplaca gen. nov. Teloschistacearum (Flechten des Himalaya 16)". Khumbu Himal, Ergebnisse des Forschungsunternehemens Nepal Himalaya (in German). 6: 443–446.
- Kondratyuk, S.Y.; Lőkös, L.; J.A., Kim; Kondratiuk, A.S.; Jeong, M.H.; Jang, S.H.; Oh, S.O.; Hur, J.S. (2015). "Three new monotypic genera of the caloplacoid lichens (Teloschistaceae, lichen-forming ascomycetes)". Mycobiology. 43 (3): 195–202. doi:10.5941/MYCO.2015.43.3.195. PMC 4630424. PMID 26539034.
- Kondratyuk et al. 2017, p. 107.
- Trevisan, V. (1857). "Nuovi studi sui licheni spettanti alle tribù delle Patellariee, Baeomycee e Lecideinee" [New studies on lichens pertaining to the tribes Patellariaceae, Baeomycetaceae, and Lecideaceae]. Revista Periodica dei Lavori della Imperiale Regia Accademia di Padova (in Italian). 5: 63–79.
- Bungartz, Søchting & Arup 2020, p. 534.
- Kondratyuk et al. 2017, p. 108.
- Hue, A. (1887). "Addenda nova ad Lichenographiam europaeam. Exposuit in Flora Ratisbonensi Dr. W. Nylander, in ordine vero systematico deposuit. Pars II" [New additions to the European lichenography. Presented in the Regensburg Flora by Dr. W. Nylander, arranged in systematic order. Part II]. Revue de Botanique (in Latin). 6: 5–192 [148].
- Bungartz, F.; Søchting, U.; Arup, U. (2021). "Obscuroplaca gen. nov. – a replacement name for Phaeoplaca; Teloschistaceae (lichenized Ascomycota) from the Galapagos Islands". Plant and Fungal Systematics. 66 (2): 240–241. doi:10.35535/pfsyst-2021-0022. S2CID 245619926.
- Bungartz, Søchting & Arup 2020, p. 540.
- Kondratyuk, S.Y.; Persson, P.-E.; Hansson, M.; Lőkös, L.; Kondratiuk, A.S.; Fayyaz, I; Kouser, R.; Afshan, N.S.; Niazi, A.R.; Zulfiqar, R.; Khalid, A.N.; Kärnefelt, I.; Farkas, E.; Hur, J.-S.; Thell, A. (2022). "Contributions to Molecular Phylogeny of Lichens 4. New names in the Teloschistaceae". Acta Botanica Hungarica. 64 (3–4): 313–336. doi:10.1556/034.64.2022.3-4.7. S2CID 253796366.
- Kondratyuk et al. 2017, p. 112.
- Kondratyuk et al. 2017, p. 113.
- Massalongo, A.B. (1852). "Monografia dei licheni blasteniospori" [Monograph of blasteniospore lichens]. Atti dell'Istituto Veneto Scienze. ser 2 (in Italian) (3(App. 3)): 5–131 [119].
- Arup, Søchting & Frödén 2013, p. 74.
- Poelt, J. (1983). "Musterbeispiele analoger Lagerdifferenzierung bei Flechten: Almbornia, Speerschneidera, Seirophora gen. nov" [Exemplary cases of analog differential storage in lichens: Almbornia, Speerschneidera, Seirophora gen nov.]. Flora (in German). 174 (5/6): 439–445. doi:10.1016/S0367-2530(17)31400-7.
- Bungartz, Søchting & Arup 2020, p. 548.
- Arup, Søchting & Frödén 2013, p. 75.
- Kondratyuk et al. 2017, p. 118.
- Wilk et al. 2021, pp. 292–293.
- Kondratyuk et al. 2013, p. 265.
- Wilk et al. 2021, pp. 293–294.
- Kondratyuk et al. 2017, p. 86.
- Kondratyuk et al. 2013, p. 267.
- Dodge, C.W. (1966). "New lichens from Chile". Nova Hedwigia. 12: 307–352.
- Kondratyuk et al. 2013, p. 268.
- Wilk et al. 2021, p. 284.
- Arup, Søchting & Frödén 2013, p. 61.
- Kondratyuk et al. 2017, p. 96.
- Kondratyuk, S.Y.; Persson, P.-E.; Hansson, M.; Lőkös, L.; Liu, D.; Hur, J.-S.; Kärnefelt, I.; Thell, A. (2018). "Hosseusiella and Rehmanniella, two new genera in the Teloschistaceae" (PDF). Acta Botanica Hungarica. 60 (1–2): 89–113. doi:10.1556/034.60.2018.1-2.7.
- Kondratyuk et al. 2017, p. 105.
- Fayyaz, Iram; Kouser, Rubina; Afshan, Najam-ul-Sehar; Niazi, Abdul Rehman; Zulfiqar, Rizwana; Khalid, Abdul Nasir; Kondratyuk, Sergey Yakovych (2022). "Iqbalia kashmirensis gen. et sp. nov. from Pakistan (Teloschistaceae, lichenized ascomycetes) based on multigene phylogeny". Mycological Progress. 21 (8): 1–15. doi:10.1007/s11557-022-01823-y. S2CID 251031210.
- Kondratyuk, S.; Kärnefelt, I. (1997). "Josefpoeltia and Xanthomendosa, two new genera in the Teloschistaceae (lichenized Ascomycotina)". Bibliotheca Lichenologica. 68: 19–44.
- Kondratyuk et al. 2013, p. 269.
- Kondratyuk et al. 2017, p. 109.
- Kondratyuk, S.Y.; Lőkös, L.; Farkas, E.; Jang, S.-H.; Liu, D.; Halda, J.; Persson, P.-E.; Hansson, M.; Kärnefelt, I.; Thell, A.; Fačkovcová, Z.; Yamamoto, Y.; Hur, J.-S. (2019). "New and noteworthy lichen-forming and lichenicolous fungi 9". Acta Botanica Hungarica. 61 (3–4): 325–367. doi:10.1556/034.61.2019.3-4.6. S2CID 229283962.
- Kondratyuk et al. 2015, p. 330.
- Kondratyuk et al. 2017, p. 111.
- Massalongo, A. (1861). "Lichenes capenses quos collegit in itinere 1853–1856 Dr H. Wavra, a Dott. A. Massalongo delineati ac descripti" [Lichens of the Cape that were collected during the journey of 1853–1856 by Dr. H. Wavra, depicted and described by Dr. A. Massalongo]. Mem. Dell' Istituto Veneto di Scienze, Lett. Ed Arti. 10: 84.
- Kondratyuk et al. 2015b, p. 331.
- Wilk et al. 2021, p. 287.
- Arup, Søchting & Frödén 2013, p. 62.
- Arup, Søchting & Frödén 2013, p. 64.
- Kondratyuk et al. 2015b, p. 334.
- Kondratyuk et al. 2015b, p. 335.
- Wilk et al. 2021, pp. 279, 284, 287, 294.
- Kondratyuk, Sergey Y.; Kärnefelt, Ingvar; Thell, Arne; Elix, John A.; Kim, Jung; Kondratiuk, Anna S.; Hur, Jae-Seoun (2015). "Tassiloa, a new genus in the Teloschistaceae (lichenized ascomycetes)". Graphis Scripta. 27 (1–2): 22–26.
- Wilk et al. 2021, pp. 279, 287, 294.
- Arup, Søchting & Frödén 2013, p. 65.
- Kondratyuk et al. 2015b, p. 337.
- Arup, Søchting & Frödén 2013, p. 66.
- Kondratyuk, S.Y.; Mosyakin, S.L. (2022). "Wilketalia S.Y.Kondr., a new name for Andina Wilk, Pabijan & Lücking, nom. illeg. (Teloschistaceae, lichenized Ascomycota)". Ukrainian Botanical Journal. 79 (1): 3–5. doi:10.15407/ukrbotj79.01.003. S2CID 247535880.
- Søchting, Ulrik; Garrido-Benavent, Isaac; Seppelt, Rod; Castello, Miris; Pérez-Ortega, Sergio; De Los Ríos Murillo, Asunción; Sancho, Leopoldo Garcia; Frödén, Patrik; Arup, Ulf (2014). "Charcotiana and Amundsenia, two new genera in Teloschistaceae (lichenized Ascomycota, subfamily Xanthorioideae) hosting two new species from continental Antarctica, and Austroplaca frigida, a new name for a continental Antarctic species". The Lichenologist. 46 (6): 763–782. doi:10.1017/S0024282914000395. S2CID 86840341.
- Arup, Søchting & Frödén 2013, p. 34.
- Arup, Søchting & Frödén 2013, p. 37.
- Arup, Søchting & Frödén 2013, p. 38.
- Arup, Søchting & Frödén 2013, p. 40.
- Kondratyuk, S.Y.; Kärnefelt, I.; Lőkös, L.; Hur, J.S.; Thell, A. (2018). "Coppinsiella and Seawardiella – two new genera of the Xanthorioideae (Teloschistaceae, lichen-forming Ascomycota)" (PDF). Acta Botanica Hungarica. 60 (3–4): 369–386. doi:10.1556/034.60.2018.3-4.8. S2CID 91496354.
- Kondratyuk et al. 2017, p. 79.
- Luyken, J.A. (1809). Tentamen historiae lichenum [A Study of Lichen History] (in Latin). Gottingen. p. 93.
- Arup, Søchting & Frödén 2013, p. 44.
- Kondratyuk et al. 2017, p. 87.
- Fedorenko, Natalya M.; Stenroos, Soili; Thell, Arne; Kärnefelt, Ingvar; Elix, John A.; Hur, Jae-Seoun; Kondratyuk, Sergij Y. (2012). "Molecular phylogeny of xanthorioid lichens (Teloschistaceae, Ascomycota), with notes on their morphology". In Kärnefelt, Ingvar; Seaward, Mark R.D.; Thell, Arne (eds.). Systematics, biodiversity and ecology of lichens. Bibliotheca Lichenologica. Vol. 108. pp. 45–64. ISBN 978-3-443-58087-2.
- Kondratyuk, S.Y.; Kärnefelt, I.; Thell, A.; Elix, J.A.; Kim, J.; Jeong, M.H.; Yu, N.N.; Kondratiuk, A.S.; Hur, J.S. (2014). "A revised taxonomy of the subfamily Xanthorioideae (Teloschistaceae, Ascomycota) based on molecular phylogeny". Acta Botanica Hungarica. 56 (1–2): 141–178. doi:10.1556/abot.56.2014.1-2.12.
- Arup, Søchting & Frödén 2013, p. 31.
- Kondratyuk et al. 2017, p. 101.
- Bungartz, Søchting & Arup 2020, p. 527.
- Kondratyuk et al. 2015b, p. 329.
- Arup, Søchting & Frödén 2013, p. 47.
- Fedorenko, Natalya M.; Stenroos, Soili; Thell, Arne; Kärnefelt, Ingvar; Kondratyuk, Sergey Y. (2009). "A phylogenetic analysis of xanthorioid lichens (Teloschistaceae, Ascomycota) based on ITS and mtSSU sequences". In Thell, Arne; Seaward, Mark; Feuerer, Tassilo (eds.). Diversity of Lichenology – Anniversary Volume. Bibliotheca Lichenologica. Vol. 100. J. Cramer in der Gebrüder Borntraeger Verlagsbuchhandlung. pp. 49–84. ISBN 978-3-443-58079-7.
- Kondratyuk, S.Y.; Kärnefelt, I. (2003). "Revision of three natural groups of xanthorioid lichens (Teloschistaceae, Ascomycota)". Ukrainskiy Botanichnyi Zhurnal. 60 (4): 427–437.
- Arup, Søchting & Frödén 2013, p. 48.
- Arup, Søchting & Frödén 2013, p. 49.
- Hue, A. (1908). "Quator lichenum exoticorum" [Four exotic lichens]. Bulletin de la Société linnéenne de Normandie. 6 (in Latin) (1): 75.
- Arup, Søchting & Frödén 2013, p. 55.
- Kondratyuk et al. 2017, p. 115.
- Kondratyuk et al. 2017, p. 117.
- Duvigneaud, P.A. (1941). "Xanthodactylon Duvign., genre nouveau de lichen de l'Afrique du Sud" [Xanthodactylon Duvign., a new genus of lichen from South Africa, related to the lichens of the genera Teloschistes and Apatoplaca (Teloschistaceae)]. Bulletin du Jardin Botanique de l'État à Bruxelles (in French). 16 (2–3): 259–265. doi:10.2307/3666548. JSTOR 3666548.
- Santesson, R. (1949). "Dolichocarpus and Xanthopeltis, two new lichen genera from Chile". Svensk Botanisk Tidskrift. 43 (2–3): 547–567.
- Kondratyuk, S.Y.; Kim, J.A.; Yu, N.-H.; Jeong, M.-H.; Jang, S.H.; Kondratiuk, A.S.; Zarei-Darki, B.; Hur, J.-S. (2015). "Zeroviella, a new genus of xanthorioid lichens (Teloschistaceae, Ascomycetes) proved by three gene phylogeny". Ukrainian Botanical Journal. 72 (6): 574–584. doi:10.15407/ukrbotj72.06.574.
- Brodo, Irwin M.; Sharnoff, Sylvia Duran; Sharnoff, Stephen (2001). Lichens of North America. Yale University Press. p. 319. ISBN 978-0-300-08249-4.
- García, Renato; Magnin, Lucia; Miotti, Laura; Barrientos, Gustavo (2020). "Lichens growing on human bone remains: A case study from continental Patagonia (Deseado Massif, Santa Cruz, Argentina)". Journal of King Saud University - Science. 32 (3): 2219–2221. doi:10.1016/j.jksus.2020.02.029. S2CID 216163263.
- Fraser, Shannon J.; Bowman, E.A.; Gianopulos, Nikolas G.; Newcombe, George (2016). "Xanthoria parietina in the Inland Pacific Northwest". North American Fungi. 11 (2): 1–12.
- Diederich, Paul; Lawrey, James D.; Ertz, Damien (2018). "The 2018 classification and checklist of lichenicolous fungi, with 2000 non-lichenized, obligately lichenicolous taxa". The Bryologist. 121 (3): 340–425 [364–366]. doi:10.1639/0007-2745-121.3.340. S2CID 92396850.
- Kantvilas, Gintaras; Suija, Ave; Motiejūnaitė, Jurga (2021). "Caloplaca tephromelae (Teloschistaceae), a new lichenicolous species from Tasmania". The Lichenologist. 53 (4): 317–325. doi:10.1017/s0024282921000207. S2CID 236502837.
- Søchting, Ulrik; Olech, Maria (1995). "The lichen genus Caloplaca in polar regions". The Lichenologist. 27 (6): 463–471. doi:10.1016/s0024-2829(95)80006-9. S2CID 83696222.
- Vondrák, Jan; Frolov, Ivan; Říha, Pavel; Hrouzek, Pavel; Palice, Zdeněk; Nadyeina, Olga; Halici, Gökhan; Khodosovtsev, Alexander; Roux, Claude (2013). "New crustose Teloschistaceae in Central Europe". The Lichenologist. 45 (6): 701–722. doi:10.1017/s0024282913000455. hdl:10995/27377. S2CID 85942896.
- Vondrák, Jan; Frolov, Ivan; Davydov, Evgeny A.; Yakovchenko, Lidia; Malíček, Jiří; Svoboda, Stanislav; Kubásek, Jiří (2019). "The lichen family Teloschistaceae in the Altai-Sayan region (Central Asia)". Phytotaxa. 396 (1): 1. doi:10.11646/phytotaxa.396.1.1. S2CID 92315392.
- Vondrák, Jan; Ismailov, Aziz; Urbanavichus, Gennadii (2017). "Lichens of the family Teloschistaceae in Dagestan, an eastern part of the Caucasian biodiversity hot-spot". Nova Hedwigia. 104 (4): 483–498. doi:10.1127/nova_hedwigia/2016/0387.
- Mishra, G.K.; Upreti, D.K.; Nayaka, S.; Thell, A.; Kärnefelt, I.; Lőkös, L.; Hur, J.-S.; Sinha, G.P.; Kondratyuk, S.Y. (2020). "Current taxonomy of the lichen family Teloschistaceae from India with descriptions of new species". Acta Botanica Hungaricae. 62 (3–4): 309–391. doi:10.1556/034.62.2020.3-4.5. S2CID 228982203.
- Bungartz, Søchting & Arup 2020.
- Herrera-Campos, Ma. de los Ángeles; Lücking, Robert; Pérez-Pérez, Rosa Emilia; Miranda-González, Ricardo; Sánchez, Norberto; Barcenas-Peña, Alejandrina; Carrizosa, Abraham; Zambrano, Angel; Ryan, Bruce D.; Nash, Thomas H. (2014). "Biodiversidad de líquenes en México" [Biodiversity of lichens in Mexico]. Revista Mexicana de Biodiversidad (in Spanish). 85: 82–99. doi:10.7550/rmb.37003.
- de Lange, Peter; Dan, Blanchon; Knight, Allison; Elix, John; Lücking, Robert; Frogley, Kelly; Harris, Anna; Cooper, Jerry; Rolfe, Jeremy (2018). Conservation status of New Zealand indigenous lichens and lichenicolous fungi, 2018 (PDF) (Report). New Zealand Threat Classification Series 27. Wellington: Department of Conservation.
- Tudor, Phoebe B.; Matero, Frank G.; Koestler, Robert J. (1990). "A Case Study of the Compatibility of Biocidal Cleaning and Consolidation in the Restoration of a Marble Statue". Biodeterioration Research. Boston, MA: Springer US. pp. 525–533. doi:10.1007/978-1-4757-9453-3_42. ISBN 978-1-4757-9455-7.
- Nayak, Sandeep Kumar; Behera1, Prashant Kumar; Bajpai, Rajesh; Upreti, Dalip Kumar; Satapathy, Kunja Bihari (2017). "Lichens growth on Sun Temple of Konark in Odisha, India- A curse or blessing". Cryptogam Biodiversity and Assessment. 2 (02): 48–52. doi:10.21756/cab.v2i02.11119.
- Crawford, Stuart (2019). "Lichens Used in Traditional Medicine". In Ranković, Branislav (ed.). Lichen Secondary Metabolites. Bioactive Properties and Pharmaceutical Potential (2 ed.). Springer Nature Switzerland AG. p. 76. ISBN 978-3-030-16813-1.
- Belguidoum, Amina; Haichour, Rima; Lograda, Takia; Ramdani, Messaoud (2022). "Biomonitoring of air pollution by lichen diversity in the urban area of Setif, Algeria". Biodiversitas Journal of Biological Diversity. 23 (2): 970–981. doi:10.13057/biodiv/d230240. S2CID 247442207.
- Brandt, Annette; de Vera, Jean-Pierre; Onofri, Silvano; Ott, Sieglinde (2014). "Viability of the lichen Xanthoria elegans and its symbionts after 18 months of space exposure and simulated Mars conditions on the ISS". International Journal of Astrobiology. 14 (3): 411–425. doi:10.1017/s1473550414000214. S2CID 53421340.
- Ravera, S. (2017). "Caloplaca rinodinae-albae". IUCN Red List of Threatened Species. 2017. Retrieved 23 August 2023.
- Sokoloff, P.; McMullin, T. (2020). "Seirophora aurantiaca". IUCN Red List of Threatened Species. 2020. Retrieved 23 August 2023.
- Ramos, D.; Vargas, R.; Herrera-Campos, M.; Moat, J.; Whaley, O.; Parrinello, C.; Bungartz, F. (2021). "Teloschistes peruensis". IUCN Red List of Threatened Species. 2021. Retrieved 23 August 2023.
- Sparkes, J.H.; de Lange, P.J.; Blanchon, D.J. (2014). "Notes on Caloplaca allanii Zahlbr. (Teloschistaceae) a poorly known West Auckland, North Island, New Zealand endemic". New Zealand Journal of Botany. 52 (3): 304–309. doi:10.1080/0028825x.2014.891240. S2CID 85673860.
- Bungartz, Søchting & Arup 2020, p. 516.
- Xinli, Wei; Hong, Deng; Jiangchun, Wei (2020). "Threatened categories assessment of lichens in China". Biodiversity Science. 28 (1): 54–65 [58]. doi:10.17520/biods.2019154. S2CID 218807267.
Cited literature
- Arup, Ulf; Søchting, Ulrik; Frödén, Patrik (2013). "A new taxonomy of the family Teloschistaceae". Nordic Journal of Botany. 31 (1): 16–83. doi:10.1111/j.1756-1051.2013.00062.x.
- Bungartz, Frank; Søchting, Ulrik; Arup, Ulf (2020). "Teloschistaceae (lichenized Ascomycota) from the Galapagos Islands: a phylogenetic revision based on morphological, anatomical, chemical, and molecular data". Plant and Fungal Systematics. 65 (2): 515–576. doi:10.35535/pfsyst-2020-0030. S2CID 234385561.
- Kondratyuk, S.; Jeong, M.-H.; Yu, N.-H.; Kärnefelt, I.; Thell, A.; Elix, J.; Kim, J.; Kondratyuk, A.; Hur, J.-S. (2013). "Four new genera of teloschistoid lichens (Teloschistaceae, Ascomycota) based on molecular phylogeny". Acta Botanica Hungarica. 55 (3–4): 251–274. doi:10.1556/abot.55.2013.3-4.8.
- Kondratyuk, S.Y.; Kärnefelt, I.; Thell, A.; Elix, J.A.; Kim, J.; Kondratiuk, A.S.; Hur, J.-S. (2015b). "Brownlielloideae, a new subfamily in the Teloschistaceae (Lecanoromycetes, Ascomycota)" (PDF). Acta Botanica Hungarica. 57 (3–4): 321–343. doi:10.1556/034.57.2015.3-4.6.
- Kondratyuk, S.Y.; Lőkös, L.; Upreti, D.K.; Nayaka, S.; Mishra, G.K.; Ravera, S.; Jeong, M.-H.; Jang, S.-H.; Park, J.S.; Hur, J.S. (2017). "New monophyletic branches of the Teloschistaceae (lichen-forming Ascomycota) proved by three gene phylogeny". Acta Botanica Hungarica. 59 (1–2): 71–136. doi:10.1556/034.59.2017.1-2.6. hdl:10447/414429.
- Wilk, Karina; Pabijan, Maciej; Saługa, Marta; Gaya, Ester; Lücking, Robert (2021). "Phylogenetic revision of South American Teloschistaceae (lichenized Ascomycota, Teloschistales) reveals three new genera and species". Mycologia. 113 (2): 278–299. doi:10.1080/00275514.2020.1830672. PMID 33428561. S2CID 231586897.

_Th._Fr_1165920.jpg.webp)

_Hellb..jpg.webp)



.jpg.webp)










.jpg.webp)

