Allethrins
The allethrins are a group of related synthetic compounds used in insecticides. They are classified as pyrethroids, i.e. synthetic versions of pyrethrin, a chemical with insecticidal properties found naturally in Chrysanthemum flowers. They were first synthesized in the United States by Milton S. Schechter in 1949. Allethrin was the first pyrethroid.
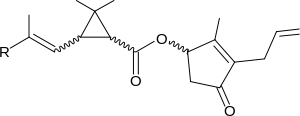
Allethrin II (R = −COOCH3)
They are commonly used in ultra-low volume sprays for outdoor mosquito control, and in many household insecticides such as RAID, as well as mosquito coils.
Chemical structure
Allethrin I and allethrin II differ by having a methyl group and a methyl ester, respectively, on one terminus. Each of these allethrins consists of the eight possible stereoisomers. A partly enantiopure variant of allethrin I, consisting of only two stereoisomers in an approximate ratio of 1:1, is called bioallethrin. The same mixture of isomers, but in an approximate ratio of 3:1, is known as esbiothrin.
Toxicity
Chronic exposure to allethrins alters the plasma biochemical profile of humans and may have adverse health effects.[1] Bioallethrin has been shown to cause oxidative damage, cellular toxicity and necrosis of human lymphocytes studied in vitro.[2] It is highly toxic to fish and aquatic invertebrates. At normal application rates, allethrin is slightly toxic to bees.[3] Insects subject to exposure become paralyzed (nervous system effect) before dying. Allethrins are toxic to cats[4] because they either do not produce, or produce less of certain isoforms of glucuronosyltransferase, which serve in hepatic detoxifying metabolism pathways.[5]
Notes
- Narendra, M.; Kavitha, G.; Helah Kiranmai, A.; Raghava Rao, N.; Varadacharyulu, N.C. (September 2008). "Chronic exposure to pyrethroid-based allethrin and prallethrin mosquito repellents alters plasma biochemical profile". Chemosphere. 73 (3): 360–364. Bibcode:2008Chmsp..73..360N. doi:10.1016/j.chemosphere.2008.05.070. PMID 18657844.
- Arif, Amin; Quds, Ruhul; Mahmood, Riaz (December 2021). "Bioallethrin enhances generation of ROS, damages DNA, impairs the redox system and causes mitochondrial dysfunction in human lymphocytes". Scientific Reports. 11 (1): 8300. doi:10.1038/s41598-021-87799-3. PMC 8050322. PMID 33859309.
- "Pesticide Information Profile - Allethrin". pmep.cce.cornell.edu.
- "Pyrethrin and Permethrin Toxicity in Dogs and Cats". peteducation.com. Archived from the original on 2013-05-25. Retrieved 2013-05-12.
- Court, M. H.; Greenblatt, D. J. (2000). "Molecular genetic basis for deficient acetaminophen glucuronidation by cats: UGT1A6 is a pseudogene, and evidence for reduced diversity of expressed hepatic UGT1A isoforms". Pharmacogenetics. 10 (4): 355–369. doi:10.1097/00008571-200006000-00009. PMID 10862526.
References
- Oregon State University (1996). Allethrin. Retrieved October 26, 2005.
- Illinois Department of Public Health Pyrethroid Insecticides Fact Sheet. Retrieved October 26, 2005.
- World Health Organization (WHO) d-Allethrin. Retrieved October 26, 2005.
- Jim E. Riviere & Mark G. Papich Eds.: Veterinary Pharmacology and Therapeutics. Iowa State University Press, 2009. ISBN 9780813820613. (p. 1194)