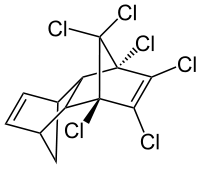
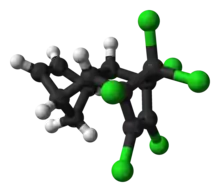
Aldrin
Aldrin is an organochlorine insecticide that was widely used until the 1990s, when it was banned in most countries. Aldrin is a member of the so-called "classic organochlorines" (COC) group of pesticides. COCs enjoyed a very sharp rise in popularity during and after The Second World War. Other noteworthy examples of COCs include DDT.[3] After research showed that organochlorines can be highly toxic to the ecosystem through bioaccumulation, most were banned from use. It is a colourless solid. Before the ban, it was heavily used as a pesticide to treat seed and soil. Aldrin and related "cyclodiene" pesticides (a term for pesticides derived from Hexachlorocyclopentadiene) became notorious as persistent organic pollutants.[4]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(1R,4S,4aS,5S,8R,8aR)-1,2,3,4,10,10-Hexachloro-1,4,4a,5,8,8a-hexahydro-1,4:5,8-dimethanonaphthalene | |
| Other names | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.652 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 2762, 2761 |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C12H8Cl6 |
| Molar mass | 364.90 g·mol−1 |
| Appearance | Colorless solid |
| Density | 1.60 g/mL[1] |
| Melting point | 104 °C (219 °F; 377 K) |
Solubility in water |
slightly soluble (0.003%)[1] |
| Vapor pressure | 7.5 × 10−5 mmHg @ 20 °C |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
potential occupational carcinogen[1] |
| GHS labelling: | |
Pictograms |
   |
Signal word |
Danger |
Hazard statements |
H300, H301, H310, H311, H351, H372, H410 |
Precautionary statements |
P201, P202, P260, P262, P264, P270, P273, P280, P281, P301+P310, P302+P350, P302+P352, P308+P313, P310, P312, P314, P321, P322, P330, P361, P363, P391, P405, P501 |
| NFPA 704 (fire diamond) | |
| Flash point | 66 °C (151 °F; 339 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
50 mg/kg (rabbit, oral) 33 mg/kg (guinea pig, oral) 39 mg/kg (rat, oral) 44 mg/kg (mouse, oral)[2] |
LCLo (lowest published) |
5.8 mg/m3 (rat, 4 hr)[2] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
TWA 0.25 mg/m3 [skin][1] |
REL (Recommended) |
Ca TWA 0.25 mg/m3 [skin][1] |
IDLH (Immediate danger) |
25 mg/m3[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Structure & Reactivity
The structure formula of aldrin is C12H8Cl6. The molecule has a molecular weight of 364.896 g/mol. The melting point of aldrin is a temperature of 105 °C and the octanol-water partition coefficient is 6.5 (logP).[5]
Pure aldrin takes form as a white crystalline powder. Though it is not soluble in water (0.003%% solubility), aldrin dissolves very well in organic solvents, such as ketones and paraffins.[6] Aldrin decays very slowly once released into the environment. Though it is rapidly converted to dieldrin by plants and bacteria, dieldrin maintains the same toxic effects and slow decay of aldrin.[7] Aldrin is easily transported through the air by dust particles. Aldrin does not react with mild acids or bases and is stable in an environment with a pH between 4 and 8. It is highly flammable when exposed to temperatures above 200 °C[5] In the presence of oxidizing agents aldrin reacts with concentrated acids and phenols.
Synthesis
Aldrin is not formed in nature. It is synthesized by combining hexachlorocyclopentadiene with norbornadiene in a Diels-Alder reaction to give the adduct.[8] In 1967, the composition of technical-grade aldrin was reported to consist of 90.5% of hexachlorohexahydrodimethanonaphthalene (HHDN).[7]
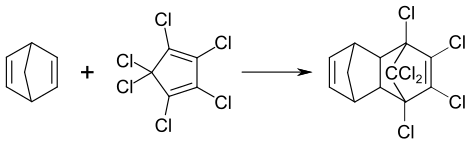
Similarly, an isomer of aldrin, known as isodrin, is produced by reaction of hexachloronobornadiene with cyclopentadiene.[9] Isodrin is also produced as a byproduct of aldrin synthesis, with technical-grade aldrin containing about 3,5% isodrin.[7]
Aldrin is named after the German chemist Kurt Alder, one of the coinventors of this kind of reaction. An estimated 270 million kilograms of aldrin and related cyclodiene pesticides were produced between 1946 and 1976.
Available forms
There are multiple available forms of aldrin. One of these is the isomer isodrin, which cannot be found in nature, but needs to be synthesized like aldrin. When aldrin enters the human body or the environment it is rapidly converted to dieldrin. Degradation by ultraviolet radiation or microbes can convert dieldrin to photodieldrin and aldrin to photoaldrin.[7]
Mechanism of action
Even though many toxic effects of aldrin have been discovered, the exact mechanisms underlying the toxicity are yet to be determined. The only toxic aldrin induced process that is largely understood is that of neurotoxicity.
Neurotoxicity
One of the effects that intoxication with aldrin gives rise to is neurotoxicity. Studies have shown that aldrin stimulates the central nervous system (CNS), which may cause hyperexcitation and seizures.[10] This phenomenon exerts its effect through two different mechanisms.
One of the mechanisms uses the ability of aldrin to inhibit brain calcium ATPases.[11] These ion pumps relieve the nerve terminal from calcium by actively pumping it out. However, when aldrin inhibits these pumps, the intracellular calcium levels rise. This results in an enhanced neurotransmitter release.
The second mechanism makes use of aldrin's ability to block gamma-aminobutyric acid (GABA) activity.[12] GABA is a major inhibitory neurotransmitter in the central nervous system. Aldrin induces neurotoxic effects by blocking the GABAA receptor-chloride channel complex. By blocking this receptor, chloride is unable to move into the synapse, which prevents hyperpolarization of neuronal synapses. Therefore, the synapses are more likely to generate action potentials.
Metabolism
The metabolism of oral aldrin exposure has not been studied in humans. However, animal studies are able to provide an extensive overview of the metabolism of aldrin. This data can be related to humans.
Biotransformation of aldrin starts with epoxidation of aldrin by mixed-function oxidases (CYP-450),[13] which forms dieldrin. This conversion happens mainly in the liver. Tissues with low CYP-450 expression use the prostaglandin endoperoxide synthase (PES) instead.[14] This oxidative pathway bisdioxygenises the arachidonic acid to prostaglandin G2 (PGG2). Subsequently, PGG2 is reduced to prostaglandin H2 (PGH2) by hydroperoxidase.
Dieldrin can then be directly oxidized by cytochrome oxidases, which forms 9-hydroxydieldrin. An alternative for oxidation involves the opening of the epoxied ring by epoxied hydrases, which forms the product 6,7-trans-dihydroxydihydroaldrin.[15] Both products can be conjugated to form 6,7-trans-dihydroxydihydroaldrin glucuronide and 9-hydroxydieldrin glucuronide, respectively. 6,7-trans-dihydroxydihydroaldrin can also be oxidized to form aldrin dicarboxylic acid.[16][17]
Efficacy and side effects
Considering the toxicokinetics of aldrin in the environment, the efficacy of the compound has been determined. In addition, the adverse effects after exposure to the aldrin are demonstrated, indicating the risk regarding the compound.
Efficacy
The ability of aldrin, in its use for the control of termites, is examined in order to determine the maximum response when applied. In 1953 US researchers tested aldrin and dieldrin on terrains with rats known to carry chiggers, at a rate of 2.25 pound per acre. The Aldrin and Dieldrin treatment demonstrated a decrease of 75 times less chiggers on rats for Dieldrin treated terrains and 25 times less chiggers on the rats when treated with Aldrin. The Aldrin treatment indicate a high productivity, especially in comparison to other insecticide that were used, like DDT, Sulfur or BHC.[18]
Adverse effects
Exposure of Aldrin to the environment leads to the localization of the chemical compound in the air, soil, and water.[7] Aldrin gets changed quickly to dieldrin and that compound degrades slowly, which accounts for aldrin concentrations in the environment around the primary exposure and in the plants.[19] These concentrations can also be found in animals, which eat contaminated plants or animals that reside in the contaminated water. This biomagnification can lead to a high concentrations in their fat.
There are some reported cases of workers who developed anemia after multiple dieldrin exposures. However the main adverse effect of Aldrin and Dieldrin is in relationship to the central nervous system.[7] The accumulated levels of Dieldrin in the body were believed to lead to convulsions.[20] Besides that other symptoms were also reported like headaches, nausea and vomiting, anorexia, muscle twitching and myoclonic jerking and EEG distortions. In all these cases removal of the source of exposure to aldrin/dieldrin led to a rapid recovery.[21]
Toxicity
The toxicity of aldrin and dieldrin is determined by the results of several animal studies. Reports of a significant increase in workers death in relation to aldrin has not been found, although death by anemia is reported in some cases after multiple exposure to Aldrin. Immunological tests linked an antigenic response to erythrocytes coated with dieldrin in those cases.[22] Direct dose-response relations being a cause for death are yet to be examined.
The NOAEL that was derived from rat studies:[7]
- The minimal risk level at acute oral exposure to Aldrin is 0.002 mg/kg/day.
- The minimal risk level at intermediate exposure to Dieldrin is 0.0001 mg/kg/day.
- The minimal risk level at chronic exposure to Aldrin is 0.00003 mg/kg/day.
- The minimal risk level at chronic exposure to Dieldrin is 0.00005 mg/kg/day.
In addition to these studies, breast cancer risk studies were performed demonstrating a significant increased breast cancer risk. After comparing blood concentrations to number of lymph nodes and tumor size a 5-fold higher risk of death was determined, comparing the highest quartile range in the research to the lower quartile range.[23] Young children are also more susceptible to the drug, causing severe generalized convulsions.[24]
Effects on animals
Most of the animal studies done with aldrin and dieldrin used rats. High doses of aldrin and dieldrin demonstrated neurotoxicity, but in multiple rat studies also showed a unique sensitivity of the mouse liver to dieldrin induced hepatocarcinogenicity.[25] Furthermore, Aldrin treated rats demonstrated an increased post-natal mortality, in which adults showed an increased susceptibility to the compounds compared to children in rats.[26]
Environmental impact and regulation
Like related polychlorinated pesticides, aldrin is highly lipophilic. Its solubility in water is only 0.027 mg/L, which exacerbates its persistence in the environment. It was banned by the Stockholm Convention on Persistent Organic Pollutants. In the U.S., aldrin was cancelled in 1974. The substance is banned from use for plant protection by the EU.[27]
Safety and environmental aspects
Aldrin has rat LD50 of 39 to 60 mg/kg (oral in rats). For fish however, it is extremely toxic, with an LC50 of 0.006 – 0.01 for trout and bluegill.[4]
In the US, aldrin is considered a potential occupational carcinogen by the Occupational Safety and Health Administration and the National Institute for Occupational Safety and Health; these agencies have set an occupational exposure limit for dermal exposures at 0.25 mg/m3 over an eight-hour time-weighted average.[28] Further, an IDLH limit has been set at 25 mg/m3, based on acute toxicity data in humans to which subjects reacted with convulsions within 20 minutes of exposure.[29]
It is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act (42 U.S.C. 11002), and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.[30]
References
- NIOSH Pocket Guide to Chemical Hazards. "#0016". National Institute for Occupational Safety and Health (NIOSH).
- "Aldrin". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- Zitko, Vladimir (2003), "Chlorinated Pesticides: Aldrin, DDT, Endrin, Dieldrin, Mirex", in Fiedler, H. (ed.), Persistent Organic Pollutants, The Handbook of Environmental Chemistry, vol. 3O, Springer Berlin Heidelberg, pp. 47–90, doi:10.1007/10751132_4, ISBN 9783540479321
- Robert L. Metcalf "Insect Control" in Ullmann’s Encyclopedia of Industrial Chemistry" Wiley-VCH, Weinheim, 2002. doi:10.1002/14356007.a14_263
- PubChem. "Aldrin". pubchem.ncbi.nlm.nih.gov. Retrieved 2019-04-06.
- "Aldrin (PIM 573)". www.inchem.org. Retrieved 2019-04-06.
- "Toxicological profile for aldrin/dieldrin" (PDF).
- Jubb, A. H. (1975). Basic Organic Chemistry, Part 5 Industrial products. London: Wiley. ISBN 0-471-85014-4.
- Bird, C. W.; Cookson, R. C.; Crundwell, E. (1961). "946. Cyclisations and rearrangements in the isodrin?aldrin series". Journal of the Chemical Society (Resumed): 4809–4816. doi:10.1039/JR9610004809.
- Gupta (May 1975). "Neurotoxicity of chronic chlorinated hydrocarbon insecticide poisoning: A clinical and electroencephalographic study in man". The Indian Journal of Medical Research. 63 (4): 601–606. PMID 55381.
- Mehrotra, B. D.; Ravichandra Reddy, S.; Desaiah, D. (1988). "Effect of subchronic dieldrin treatment on calmodulin-regulated Ca2+ pump activity in rat brain". Journal of Toxicology and Environmental Health. 25 (4): 461–469. doi:10.1080/15287398809531224. ISSN 0098-4108. PMID 2974087.
- Glotfelty, Dwight E. (1978-09-01). "The Atmosphere as a Sink for Applied Pesticides". Journal of the Air Pollution Control Association. 28 (9): 917–921. doi:10.1080/00022470.1978.11490579. ISSN 0002-2470.
- Wong, D. T.; Terriere, L. C. (March 1965). "Epoxidation of aldrin, isodrin, and heptachlor by rat liver microsomes". Biochemical Pharmacology. 14 (3): 375–377. doi:10.1016/0006-2952(65)90210-8. ISSN 0006-2952. PMID 14314340.
- Lang, B.; Frei, K.; Maier, P. (1986-10-15). "Prostaglandin synthase dependent aldrin epoxidation in hepatic and extrahepatic tissues of rats". Biochemical Pharmacology. 35 (20): 3643–3645. doi:10.1016/0006-2952(86)90640-4. ISSN 0006-2952. PMID 3094543.
- Iatropoulos, M. J. (December 1975). "Absorption, transport and organotropism of dichlorobiphenyl (DCB), dieldrin, and hexachlorobenzene (HCB) in rats". Environmental Research. 10 (3): 384–389. Bibcode:1975ER.....10..384I. doi:10.1016/0013-9351(75)90033-X. ISSN 0013-9351. PMID 1213019.
- Baldwin, M. K.; Robinson, J.; Parke, D. V. (June 1972). "A comparison of the metabolism of HEOD (dieldrin) in the CF1 mouse with that in the CFE rat". Food and Cosmetics Toxicology. 10 (3): 333–351. doi:10.1016/S0015-6264(72)80252-9. ISSN 0015-6264. PMID 5045677.
- Hutson, D. H. (December 1976). "Comparative metabolism of dieldrin in the rat (CFE) and in two strains of mouse (CF1 and LACG)". Food and Cosmetics Toxicology. 14 (6): 577–591. doi:10.1016/S0015-6264(76)80012-0. ISSN 0015-6264. PMID 1017774.
- Traub, Robert; Newson, Harold D.; Walton, Bryce C.; Audy, J. R. (1954-06-01). "Efficacy of Dieldrin and Aldrin in Area Control of the Chigger Vectors of Scrub Typhus". Journal of Economic Entomology. 47 (3): 429–435. doi:10.1093/jee/47.3.429. ISSN 1938-291X.
- Castro, Teresita F.; Yoshida, Tomio. (November 1971). "Degradation of organochlorine insecticides in flooded soils in the Philippines". Journal of Agricultural and Food Chemistry. 19 (6): 1168–1170. doi:10.1021/jf60178a041. ISSN 0021-8561. PMID 5132645.
- Versteeg, J. P. J.; Jager, K. W. (1973-04-01). "Long-term occupational exposure to the insecticides aldrin dieldrin, endrin, and telodrin". Occupational and Environmental Medicine. 30 (2): 201–202. doi:10.1136/oem.30.2.201. ISSN 1351-0711. PMC 1009505. PMID 4703092.
- Avar, P.; Czegledi-Janko, G. (1970-07-01). "Occupational exposure to aldrin: clinical and laboratory findings". Occupational and Environmental Medicine. 27 (3): 279–282. doi:10.1136/oem.27.3.279. ISSN 1351-0711. PMC 1009144. PMID 4194425.
- Krzystyniak, Krzystof; Hugo, Patrice; Flipo, Denis; Fournier, Michel (September 1985). "Increased susceptibility to mouse hepatitis virus 3 of peritoneal macrophages exposed to dieldrin". Toxicology and Applied Pharmacology. 80 (3): 397–408. doi:10.1016/0041-008x(85)90384-9. ISSN 0041-008X. PMC 7173191. PMID 2994259.
- Høyer, Annette Pernille; Jørgensen, Torben; Brock, John W.; Grandjean, Philippe (March 2000). "Organochlorine exposure and breast cancer survival". Journal of Clinical Epidemiology. 53 (3): 323–330. doi:10.1016/s0895-4356(99)00165-1. ISSN 0895-4356. PMID 10760644.
- MATSUSHITA, Toshio; TADOKORO, Yasuo (1975). "An Electroencephalographic Study for Chronic Nitroglycol Poisoning". Industrial Health. 13 (4): 237–241. doi:10.2486/indhealth.13.237. ISSN 0019-8366.
- Black, A. M. S. (November 1974). "Self Poisoning with Dieldrin: A Case Report and Pharmacokinetic Discussion". Anaesthesia and Intensive Care. 2 (4): 369–374. doi:10.1177/0310057x7400200413. ISSN 0310-057X. PMID 4614682.
- Virgo, Bruce B.; Bellward, Gail D. (October 1975). "Effect of Dietary Dieldrin on the Liver and Drug Metabolism in the Female Swiss-Vancouver Mouse". Canadian Journal of Physiology and Pharmacology. 53 (5): 903–911. doi:10.1139/y75-124. ISSN 0008-4212. PMID 1201496.
- Chemicals Regulation Directorate. "Banned and Non-Authorised Pesticides in the United Kingdom". Retrieved 1 December 2009.
- Centers for Disease Control and Prevention (4 April 2011). "Aldrin". NIOSH Pocket Guide to Chemical Hazards. Retrieved 13 November 2013.
- Centers for Disease Control and Prevention (May 1994). "Aldrin". Documentation for Immediately Dangerous To Life or Health Concentrations (IDLHs). Retrieved 13 November 2013.
- "40 C.F.R.: Appendix A to Part 355—The List of Extremely Hazardous Substances and Their Threshold Planning Quantities" (PDF) (July 1, 2008 ed.). Government Printing Office. Archived from the original (PDF) on February 25, 2012. Retrieved October 29, 2011.
{{cite journal}}: Cite journal requires|journal=(help)
