Bulevirtide
Bulevirtide, sold under the brand name Hepcludex, is an antiviral medication for the treatment of chronic hepatitis D (in the presence of hepatitis B).[3]
 | |
| Clinical data | |
|---|---|
| Trade names | Hepcludex |
| Other names | MyrB, Myrcludex-B[1] |
| License data | |
| Routes of administration | Subcutaneous |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| DrugBank | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C248H355N65O72 |
| Molar mass | 5398.951 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
The most common side effects include raised levels of bile salts in the blood and reactions at the site of injection.[3]
Bulevirtide works by attaching to and blocking a receptor (target) through which the hepatitis delta and hepatitis B viruses enter liver cells.[3] By blocking the entry of the virus into the cells, it limits the ability of HDV to replicate and its effects in the body, reducing symptoms of the disease.[3]
Bulevirtide was approved for medical use in the European Union in July 2020.[3]
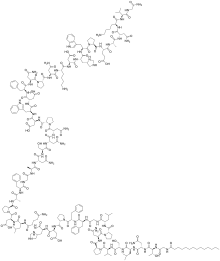
Structural formula
Bulevirtide is a 47-amino acid peptide with the following sequence:[4]
CH3(CH2)12CO-Gly-Thr-Asn-Leu-Ser-Val-Pro-Asn-Pro-Leu-Gly-Phe-Phe-Pro-Asp-His-Gln-Leu-Asp-Pro-Ala-Phe-Gly-Ala-Asn-Ser-Asn-Asn-Pro-Asp-Trp-Asp-Phe-Asn-Pro-Asn-Lys-Asp-His-Trp-Pro-Glu-Ala-Asn-Lys-Val-Gly-NH2 (C13H27CO-GTNLSVPNPLGFFPDHQLDPAFGANSNNPDWDFNPNKDHWPEANKVG-NH2)
Medical uses
Bulevirtide is indicated for the treatment of chronic hepatitis delta virus (HDV) infection in plasma (or serum) HDV-RNA positive adult patients with compensated liver disease.[3][5]
Pharmacology
Mechanism of action
Bulevirtide binds and inactivates the sodium/bile acid cotransporter, blocking both viruses from entering hepatocytes.[6]
The hepatitis B virus uses its surface lipopeptide pre-S1 for docking to mature liver cells via their sodium/bile acid cotransporter (NTCP) and subsequently entering the cells. Myrcludex B is a synthetic N-acylated pre-S1[7][8] that can also dock to NTCP, blocking the virus's entry mechanism.[9]
The drug is also effective against hepatitis D because the hepatitis D virus is only infective in the presence of a hepatitis B virus infection.[9]
References
- Deterding, K.; Wedemeyer, H. (2019). "Beyond Pegylated Interferon-Alpha: New Treatments for Hepatitis Delta". AIDS Reviews. 21 (3): 126–134. doi:10.24875/AIDSRev.19000080. PMID 31532397. S2CID 202674681.
- "Hepcludex 2 mg powder for solution for injection - Summary of Product Characteristics (SmPC)". (emc). 30 March 2022. Retrieved 1 July 2022.
- "Hepcludex EPAR". European Medicines Agency (EMA). 26 May 2020. Retrieved 12 August 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- Sauter M, Blank A, Stoll F, Lutz N, Haefeli WE, Burhenne J (May 2021). "Intact plasma quantification of the large therapeutic lipopeptide bulevirtide". Analytical and Bioanalytical Chemistry. 413 (22): 5645–54. doi:10.1007/s00216-021-03384-7. PMC 8410713. PMID 34018034.
- "Summary of opinion: Hepcludex" (PDF). European Medicines Agency. 28 May 2020.
- Francisco, Estela Miranda (29 May 2020). "Hepcludex". European Medicines Agency. Archived from the original on 15 June 2020. Retrieved 6 August 2020.
- Volz T, Allweiss L, Ben MBarek M, Warlich M, Lohse AW, Pollok JM, et al. (May 2013). "The entry inhibitor Myrcludex-B efficiently blocks intrahepatic virus spreading in humanized mice previously infected with hepatitis B virus". Journal of Hepatology. 58 (5): 861–7. doi:10.1016/j.jhep.2012.12.008. PMID 23246506.
- Abbas Z, Abbas M (August 2015). "Management of hepatitis delta: Need for novel therapeutic options". World Journal of Gastroenterology. 21 (32): 9461–5. doi:10.3748/wjg.v21.i32.9461. PMC 4548107. PMID 26327754.
- Spreitzer H (14 September 2015). "Neue Wirkstoffe – Myrcludex B". Österreichische Apothekerzeitung (in German) (19/2015): 12.