NITD008
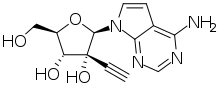
NITD008 is an antiviral drug classified as an adenosine analog (a type of nucleoside analog). It was developed as a potential treatment for flavivirus infections and shows broad spectrum antiviral activity against many related viruses such as dengue virus, West Nile virus, yellow fever virus, Powassan virus, hepatitis C virus, Kyasanur Forest disease virus, Omsk hemorrhagic fever virus, and Zika virus.[1][2][3] However, NITD008 proved too toxic in pre-clinical animal testing to be suitable for human trials, but it continues to be used in research to find improved treatments for emerging viral diseases.[4]
 | |
| Clinical data | |
|---|---|
| Trade names | NITD008 |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C13H14N4O4 |
| Molar mass | 290.279 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
See also
- Favipiravir, a drug approved by China, Germany, Indonesia, Iran, Japan, Thailand, and Turkey for treating COVID-19 patients
- MK-608, a drug with a similar structure
- Remdesivir , FDA approved antiviral drug with a similar structure
- Ribavirin, another antiviral drug with teratogenic side effects that was patented by Merck in 1971 and approved by the FDA in 1986
References
- Yin Z, Chen YL, Schul W, Wang QY, Gu F, Duraiswamy J, et al. (December 2009). "An adenosine nucleoside inhibitor of dengue virus". Proceedings of the National Academy of Sciences of the United States of America. 106 (48): 20435–9. Bibcode:2009PNAS..10620435Y. doi:10.1073/pnas.0907010106. PMC 2787148. PMID 19918064.
- Lo MK, Shi PY, Chen YL, Flint M, Spiropoulou CF (June 2016). "In vitro antiviral activity of adenosine analog NITD008 against tick-borne flaviviruses". Antiviral Research. 130: 46–9. doi:10.1016/j.antiviral.2016.03.013. PMC 5096641. PMID 27016316.
- Deng YQ, Zhang NN, Li CF, Tian M, Hao JN, Xie XP, et al. (October 2016). "Adenosine Analog NITD008 Is a Potent Inhibitor of Zika Virus". Open Forum Infectious Diseases. 3 (4): ofw175. doi:10.1093/ofid/ofw175. PMC 5063548. PMID 27747251.
- Nelson J, Roe K, Orillo B, Shi PY, Verma S (October 2015). "Combined treatment of adenosine nucleoside inhibitor NITD008 and histone deacetylase inhibitor vorinostat represents an immunotherapy strategy to ameliorate West Nile virus infection". Antiviral Research. 122: 39–45. doi:10.1016/j.antiviral.2015.07.008. PMC 4853296. PMID 26225754.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.