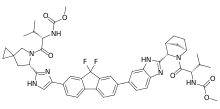
Ledipasvir
Ledipasvir is a drug for the treatment of hepatitis C that was developed by Gilead Sciences.[1] After completing Phase III clinical trials, on February 10, 2014, Gilead filed for U.S. approval of a ledipasvir/sofosbuvir fixed-dose combination tablet for genotype 1 hepatitis C.[2][3] The ledipasvir/sofosbuvir combination is a direct-acting antiviral agent that interferes with HCV replication and can be used to treat patients with genotypes 1a or 1b without PEG-interferon or ribavirin.
 | |
| Clinical data | |
|---|---|
| Trade names | Harvoni (combination with sofosbuvir) |
| Other names | GS-5885 |
| License data | |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 76% |
| Protein binding | >99% |
| Metabolism | No cytochrome metabolism |
| Elimination half-life | 47 hrs |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C49H54F2N8O6 |
| Molar mass | 889.018 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Ledipasvir is an inhibitor of NS5A, a hepatitis C virus protein.
Data presented at the 20th Conference on Retroviruses and Opportunistic Infections in March 2013 showed that a triple regimen of the nucleotide analog inhibitor sofosbuvir, ledipasvir, and ribavirin produced a 12-week post-treatment sustained virological response (SVR12) rate of 100% for both treatment-naive patients and prior non-responders with HCV genotype 1.[4][5] The sofosbuvir/ledipasvir coformulation is being tested with and without ribavirin. In February 2014 Gilead filed for United States Food and Drug Administration (FDA) approval of ledipasvir/sofosbuvir oral treatment, without interferon and ribavirin.[6]
On 10 October 2014 the FDA approved the combination product ledipasvir/sofosbuvir called Harvoni.[7]
Medical uses
Ledipasvir is most commonly used in combination with sofosbuvir for treatment in chronic hepatitis C genotype 1 patients. This drug has been tested and shown efficacy in treatment-naive and treatment experienced patients.[8]
Adverse effects
According to clinical trials, ledipasvir/sofosbuvir has been very well tolerated with the most common side effects being fatigue and headache.[9]
Interactions
Most drug-drug interactions with Harvoni involve Pgp-inducers such as St. John’s wort or rifampicin. Concomitant use will decrease the blood concentration of Harvoni and thus, have reduced therapeutic effects.[9]
Mechanism of action
Ledipasvir inhibits an important viral phosphoprotein, NS5A, which is involved in viral replication, assembly, and secretion.[10]
Sofosbuvir, on the other hand, is metabolized to a uridine triphosphate mimic, which acts as a RNA chain terminator when incorporated into RNA by NS5B polymerase.[10]
Cost
Similar to sofosbuvir, the cost of Harvoni has been a controversial topic. It costs $1,125 per pill in the US, translating to $63,000 for an 8-week treatment course, $94,500 for a 12-week treatment course, or $189,000 for a 24-week treatment course. Gilead justifies the cost by outweighing the benefit of curing hepatitis C over the cost of spending double on liver transplants or temporarily treating liver diseases. Gilead has provided a ledipasvir/sofosbuvir assistance program for eligible underserved or underinsured hepatitis C patients who cannot afford the costs of treatment.[10]
In July 2015 Gilead modified the eligibility criteria to receive Support Path benefits for HCV patients in the United States.
References
- "Ledipasvir" (PDF). United States Adopted Name. Archived from the original (PDF) on 2016-01-31. Retrieved 2013-05-14.
- "Ledipasvir-submitted-to-FDA".
- "GS-5885". Gilead Sciences. Archived from the original on 2013-04-10. Retrieved 2013-03-08.
- ELECTRON: 100% Suppression of Viral Load through 4 Weeks’ Post-treatment for Sofosbuvir + Ledipasvir (GS-5885) + Ribavirin for 12 Weeks in Treatment-naïve and -experienced Hepatitis C Virus GT 1 Patients Archived 2013-03-23 at the Wayback Machine. Gane, Edward et al. 20th Conference on Retroviruses and Opportunistic Infections. March 3–6, 2013. Abstract 41LB.
- CROI 2013: Sofosbuvir + Ledipasvir + Ribavirin Combo for HCV Produces 100% Sustained Response Archived 2015-09-24 at the Wayback Machine. Highleyman, Liz. HIVandHepatitis.com. 4 March 2013.
- "Gilead Files for U.S. Approval of Ledipasvir/Sofosbuvir Fixed-Dose Combination Tablet for Genotype 1 Hepatitis C". Gilead Sciences. 10 February 2014.
- "U.S. Food and Drug Administration Approves Gilead's Harvoni (Ledipasvir/Sofosbuvir), the First Once-Daily Single Tablet Regimen for the Treatment of Genotype 1 Chronic Hepatitis C". 10 October 2014. Archived from the original on 12 October 2018. Retrieved 10 October 2014.
- Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, et al. (May 2014). "Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection". The New England Journal of Medicine. 370 (20): 1889–98. doi:10.1056/NEJMoa1402454. PMID 24725239.
- "PRESCRIBING INFORMATION" (PDF). www.gilead.com. Retrieved 2019-06-12.
- "Ledipasvir-Sofosbuvir Harvoni - Treatment - Hepatitis C Online". www.hepatitisc.uw.edu.