Essure
Essure was a device for female sterilization. It is a metal coil which when placed into each fallopian tube induces fibrosis and blockage.[1] Essure was designed as an alternative to tubal ligation. However, it was recalled by Bayer in 2018, and the device is no longer sold due to complications secondary to its implantation. The company has reported that several patients implanted with the Essure System for Permanent Birth Control have experienced and/or reported adverse effects, including: perforation of the uterus and/or fallopian tubes, identification of inserts in the abdominal or pelvic cavity, persistent pain, and suspected allergic or hypersensitivity reaction. Although designed to remain in place for a lifetime, it was approved based on short-term safety studies. Of the 745 women with implants in the original premarket studies, 92% were followed up at one year, and 25% for two years, for safety outcomes. A 2009 review concluded that Essure appeared safe and effective based on short-term studies, that it was less invasive and could be cheaper than laparoscopic bilateral tubal ligation.[2] About 750,000 women have received the device worldwide.[3]
| Essure | |
|---|---|
 | |
| Background | |
| Type | Sterilization |
| First use | 2002 |
| Failure rates (first year, after occlusion; data disputed) | |
| Perfect use | 0.26% |
| Typical use | ? |
| Usage | |
| Duration effect | Permanent |
| Reversibility | irreversible |
| User reminders | Additional methods until 3 month check by hysterosalpingogram |
| Clinic review | None |
| Advantages and disadvantages | |
| STI protection | No |
| Benefits | Permanent contraception |
Initial trials found about 4% of people had tubal perforation, expulsion, or misplacement of the device at the time of the procedure.[3] Since 2013, the product has been controversial, with thousands of women reporting severe side effects leading to surgical extraction.[4] Rates of repeat surgery in the first year were ten times greater with Essure than with tubal ligation.[3] Campaigner Erin Brockovich has been hosting a website where women can share their stories after having the procedure.[4][5][6] As of 2015 many adverse events, including tubal perforations, intractable pain and bleeding leading to hysterectomies, possible device-related deaths, and hundreds of unintended pregnancies occurred, according to the US FDA adverse events database and other studies.[1][7]
It was developed by Conceptus Inc. and approved for use in the United States in 2002.[8] Conceptus was acquired by Bayer AG of Germany in June 2013.[9] In 2017, the CE marking in the European Union, and thus the commercial license for Essure was suspended for at least three months. Authorities in France and Ukraine recalled the implants, and the manufacturer withdrew the product voluntarily in Canada, the UK, Finland, and the Netherlands.[10][11][12][13] In April 2018, the FDA restricted sale and use of Essure which resulted in a 70% decrease in sales.[14][15] In July 2018 Bayer announced the halt of sales in the U.S. by the end of 2018.[16][17] The device is featured in the 2018 Netflix documentary The Bleeding Edge.
Use
A 2015 review found the effectiveness of Essure is unclear due to the low quality of evidence.[18] With perfect use another review found evidence of a 99.8% effective based on 5 years of follow-up.[19]
The reported insertional failure rates are "failure to place 2 inserts in the first procedure (5%), initial tubal patency (3.5%), expulsion (2.2%), perforation (1.8%), or other unsatisfactory device location (0.6%)".[20] Upon follow-up, occlusion was observed to have occurred in 96.5% of patients at 3 months with the remainder occluded by 6 months.[8] A 2015 study published in the BMJ concluded that Essure was as efficacious as laparoscopic sterilization at preventing pregnancy, but with a "10-fold higher risk of undergoing re-operation" when compared to patients who underwent a laparoscopic sterilization procedure.[7]
Follow-up
For the Essure method, three months after insertion a radiologist is supposed to perform a fluoroscopic procedure called a hysterosalpingogram,[21] to confirm that the fallopian tubes are completely blocked and that the woman can rely on the Essure inserts for birth control. A contrast agent (dye) is injected through the cervix, and an x-ray technologist takes photos of the Essure coils to ensure no contrast leaks past the Essure.[8]
Adverse effects
Serious side effects may include persistent pain, perforation of the uterus and fallopian tubes, and migration of the coils into the pelvis or abdomen.[15]
Because of the stainless steel medical staff need to be notified before magnetic resonance imaging (MRI) can be performed. However, the inserts were found to be safe with MRI using a 3-Tesla magnet and is considered MR-conditional.[22]
Procedural complications
- Inability to place inserts (4%)
- Cramping (30%)
- Pain (13%)
- Nausea/vomiting (11%)
- Dizziness/light headed (9%)
- Bleeding/spotting (7%)
- Vasovagal response (fainting) (1.3%)
- Perforation, expulsion, or other unsatisfactory location of the insert
Long-term complications[22][23]
- Abdominal pain (3.8%)
- Back pain (9%)
- Menstrual cramps, severe (2.9%)
- Pelvic or lower abdominal pain, severe (2.5%)
- Gas/bloating (1.3%)
- Headache (2.5%)
- Heavier menstrual bleeding (1.9%)
- Vaginal discharge or infection (1.5%)
- Pregnancy (0.48%)[23] and increased risk of ectopic pregnancy
- Allergic reaction to the materials
- Rash
- Autoimmune disease (0.99%)[23]
- Weight changes
- Depression
- Hair loss
- Suicide attempt (0.55%) [23]
Procedure
A physician places the coils into the fallopian tubes by a catheter passed from the vagina through the cervix and uterus.[24] This occurs successfully between 63% and 100% of the time.[25] Once in place, the ingrowth continues over a period of three months, resulting in blockage in the Fallopian tubes; the tissue barrier formed is supposed to prevent sperm from reaching an egg. During that intervening three-month period, women are advised to use an alternate contraceptive method.[24][2]
Unlike tubal ligation, it may not require a general anaesthetic (though is often done under general anaesthetic). Despite this, some women have reported considerable pain during the procedure.[26]
In one 2007 prospective study, the mean time for procedure was 6.8 minutes (range = 5–18 minutes)[24] for a trained physician to perform. The procedure can be performed in a physician's office.
The procedure is reported to be permanent and not reversible by the manufacturer. Nevertheless, several Essure reversals have been performed.
Device
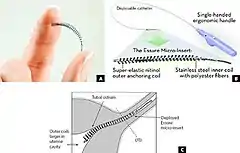
The small, flexible inserts are made from polyester fibers, nickel-titanium, stainless steel and solder. The insert contains inner polyethylene terephthalate fibers to induce inflammation, causing a benign fibrotic ingrowth,[24] and is held in place by flexible stainless steel inner coil and a dynamic outer nickel titanium alloy coil.[24] Unlike temporary methods of birth control, the Essure inserts do not contain or release hormones. The inserts do not prevent the transmission of sexually transmitted infections.[27]
Regulatory history
A Facebook group called Essure Problems which had 33,140 members (as of 04/03/2017) called the method "E-hell" and mentioned mostly pain, bleeding, bloating and other side effects from the device. Some women had coils break and perforate their internal organs, or conceived and gave birth to a child, at a number well above what Bayer has been reporting.[5] Erin Brockovich became involved in the controversy[5] and hosts a website where women can share their stories after having the procedure.[4][5] Since then Bayer provided two toll-free telephone numbers for patient complaints,[5] has advised that women reporting adverse effects are "consistent with clinical trials and consistent with what the FDA is seeing",[5] and further insisted that it wanted to hear from any women experiencing problems with Essure.[5]
In April 2015, a group of six delegates from the Essure Problems group, including a doctor with Essure experience, spoke before 36 members of the FDA and the Congressional HELP committee regarding a citizen's petition filed with the FDA. The FDA began investigating the claims of then over 16,000 members of the group as well as the legalities of the approval process that Essure went through. As of 2015, one postmarketing study was not published for 13 years after the device was approved, and another postmarketing study had not been published as of 2015.[1]
FDA
The product was approved by the FDA in 2002.[5] In 2013, the product made news in North America, with women complaining of severe side effects leading to surgical extraction. According to one article, women who have gotten pregnant are naming these children e-babies.[4]
In October 2013, the FDA stated that since the product was approved in 2002 it had received 943 reports of adverse events related to Essure, mainly for pain (606 of the complaints).[5] An additional 1,000 more complaints have been sent to the FDA in a voluntary reporting system, but physicians are not obliged to report complaints.[5]
In June 2015, the FDA reported an investigation into Essure and its over 5000 complaints, seven reported deaths, and many additional side effects, all linked to Essure, its specific chemical composition, its improper placement and its insertion. The agency announced that its Obstetrics and Gynecology Devices Panel would conduct an evidence-based review of Essure's safety in September 2015 due to the rise in adverse event reports from only 950 reports between 2002 through October 2013, to more than 4,150, or 81 percent of the total, from October 2013 to June 2015.[28]
In February 2016, the FDA issued a "black box" label to warn the public about the harmful complications associated with the use of this device and requested Bayer to conduct a new postmarket surveillance to follow 2,000 women for at least three years, comparing the effectiveness and safety of the device with other surgical contraceptive methods. Women and doctors were required to sign a decision checklist before Essure implantation, and to give consent to a test three months later to ensure the device was properly placed and functioning.[29]
In July 2020, Bayer published interim data from the FDA-mandated postmarket surveillance study comparing patients who received Essure to those who receiving a laparoscopic tubal ligation. The interim data reported the incidence of several side effects in each group. In Essure patients, chronic lower abdominal or pelvic pain occurred in 9% and abnormal bleeding in 16%, compared to 4.5% reporting pain and 10% with abnormal bleeding in the tubal ligation group. It also reported new allergic or hypersensitivity reactions in 22% of patients and no reports of new autoimmune disorders, although blinded independent verification was pending.[30] Recruitment of patients receiving Essure into the postmarket surveillance study has ceased as the device is no longer available on the US market.
References
- Dhruva SS, Ross JS, Gariepy AM (October 2015). "Revisiting Essure--Toward Safe and Effective Sterilization". The New England Journal of Medicine. 373 (15): e17. doi:10.1056/NEJMp1510514. PMID 26397951.Free full text
- Hurskainen R, Hovi SL, Gissler M, Grahn R, Kukkonen-Harjula K, Nord-Saari M, Mäkelä M (June 2010). "Hysteroscopic tubal sterilization: a systematic review of the Essure system". Fertility and Sterility. 94 (1): 16–9. doi:10.1016/j.fertnstert.2009.02.080. PMID 19409549.
- Clark NV, Endicott SP, Jorgensen EM, Hur HC, Lockrow EG, Kern ME, et al. (November 2018). "Review of Sterilization Techniques and Clinical Updates". Journal of Minimally Invasive Gynecology. 25 (7): 1157–1164. doi:10.1016/j.jmig.2017.09.012. PMID 28939482. S2CID 24849899.
- Dearhoff D (December 22, 2013). "Women report complications from Essure birth control". Chicago Tribune. Retrieved March 2, 2014.
- Morris R (June 24, 2014). "Erin Brockovich calls for end to Bayer's Essure". BBC News, Los Angeles. Retrieved June 24, 2014.
- Stein R (21 September 2015). "FDA Revisits Safety Of Essure Contraceptive Device". NPR. Retrieved 21 September 2015.
- Mao J, Pfeifer S, Schlegel P, Sedrakyan A (October 2015). "Safety and efficacy of hysteroscopic sterilization compared with laparoscopic sterilization: an observational cohort study". BMJ. 351: h5162. doi:10.1136/bmj.h5162. PMC 4604215. PMID 26462857.Free full text
- "Essure System - P020014". US Food and Drug Administration. 2009-06-29. Retrieved 2011-05-21.
- "Essure Permanent Birth Control". U.S. Food and Drug Administration. Retrieved June 24, 2014.
- Isle JW (17 August 2017). "Essure Loses Commercial License in European Union - Legal Reader". Legalreader.com. Retrieved 16 May 2018.
- "Essure Sales Halted In European Union, Implants Recalled". Birthcontrolproblems.com. 7 August 2017. Retrieved 16 May 2018.
- "EU suspends sale of contraceptive implant". Medicalspress.com. Retrieved 16 May 2018.
- "Satojen naisten sterilisaatiot menneet pieleen Suomessa – vaeltamaan lähteneitä implantteja löydetty muualta elimistöstä". Yle Uutiset. 17 August 2017. Retrieved 16 May 2018.
- US FDA Medical Devices--FDA Activities: Essure Page Last updated 04/09/2018, accessed 16 May 2018
- Office of the Commissioner (18 March 2019). "Statement from FDA Commissioner Scott Gottlieb, M.D., on manufacturer announcement to halt Essure sales in the U.S.; agency's continued commitment to postmarket review of Essure and keeping women informed". FDA. Retrieved 6 September 2019.
- Bellon T. "Bayer to phase out Essure birth control device in U.S." U.S. Retrieved 2018-07-26.
- "Sales of Essure birth control implant to be halted by Bayer; U.S. last to sell controversial device". Washington Post. Retrieved 6 September 2019.
- la Chapelle CF, Veersema S, Brölmann HA, Jansen FW (June 2015). "Effectiveness and feasibility of hysteroscopic sterilization techniques: a systematic review and meta-analysis". Fertility and Sterility. 103 (6): 1516–25.e1–3. doi:10.1016/j.fertnstert.2015.03.009. PMID 25910565.
- Ouzounelli M, Reaven NL (March 2015). "Essure hysteroscopic sterilization versus interval laparoscopic bilateral tubal ligation: a comparative effectiveness review". Journal of Minimally Invasive Gynecology. 22 (3): 342–52. doi:10.1016/j.jmig.2014.12.002. PMID 25499775.
- "Prescribing Information" (PDF). Essure. Conceptus. 2005-09-08. Archived from the original (PDF) on 2006-11-11. Retrieved 2006-12-12.
- "Essure Confirmation Test". Conceptus Inc. Archived from the original on 2011-06-12. Retrieved 2011-05-30.
- "Essure Label" (PDF). 2002. Retrieved 13 July 2020.
- Bouillon K, Bertrand M, Bader G, Lucot JP, Dray-Spira R, Zureik M (January 2018). "Association of Hysteroscopic vs Laparoscopic Sterilization With Procedural, Gynecological, and Medical Outcomes". JAMA. 319 (4): 375–387. doi:10.1001/jama.2017.21269. PMC 5833563. PMID 29362796.
- Miño M, Arjona JE, Cordón J, Pelegrin B, Povedano B, Chacon E (June 2007). "Success rate and patient satisfaction with the Essure sterilisation in an outpatient setting: a prospective study of 857 women". BJOG. 114 (6): 763–6. doi:10.1111/j.1471-0528.2007.01354.x. PMID 17516970. S2CID 29737812.
- Frietze G, Leyser-Whalen O, Rahman M, Rouhani M, Berenson AB (December 2015). "® Procedural Placement Success Rates on First Attempt". Journal of Gynecologic Surgery. 31 (6): 308–317. doi:10.1089/gyn.2015.0054. PMC 4657511. PMID 26633935.
- "Essure® |". www.essure.com. Archived from the original on 2018-07-26. Retrieved 2018-07-26.
- "FDA Activities". U.S. Food and Drug Administration. Retrieved 19 August 2015.
- "FDA takes additional action to better understand safety of Essure, inform patients of potential risks". U.S. Food and Drug Administration. February 29, 2016. Retrieved August 21, 2016.
- "522 Postmarket Surveillance Studies". Retrieved 19 July 2020.