Perflubron
Perflubron (INN/USAN, or perfluorooctyl bromide; brand name Imagent) is a contrast medium for magnetic resonance imaging, computer tomography and sonography.[1] It was approved for this use in the United States by the Food and Drug Administration in 1993.[2]
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
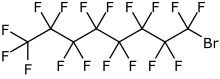
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.006.391 |
| Chemical and physical data | |
| Formula | C8BrF17 |
| Molar mass | 498.965 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.93 g/cm3 |
| Melting point | 6 °C (43 °F) |
| Boiling point | 142 °C (288 °F) |
SMILES
| |
InChI
| |
Experimental research
Perflubron has also been tested experimentally for use in liquid breathing in premature infants with respiratory distress.[3][4]

This molecular model of Perflubron is color-coded by electron density to illustrate the positive polarisation of the large bromine atom by the inductively electron-withdrawing perfluorooctyl chain.
References
- Mattrey RF (February 1989). "Perfluorooctylbromide: a new contrast agent for CT, sonography, and MR imaging". AJR. American Journal of Roentgenology. 152 (2): 247–52. doi:10.2214/ajr.152.2.247. PMID 2643258.
- FDA Approved Drug Products
- Wolfson MR, Kechner NE, Roache RF, DeChadarevian JP, Friss HE, Rubenstein SD, Shaffer TH (February 1998). "Perfluorochemical rescue after surfactant treatment: effect of perflubron dose and ventilatory frequency". Journal of Applied Physiology. 84 (2): 624–40. doi:10.1152/jappl.1998.84.2.624. PMID 9475875.
- Leach CL, Greenspan JS, Rubenstein SD, Shaffer TH, Wolfson MR, Jackson JC, et al. (September 1996). "Partial liquid ventilation with perflubron in premature infants with severe respiratory distress syndrome. The LiquiVent Study Group". The New England Journal of Medicine. 335 (11): 761–7. doi:10.1056/NEJM199609123351101. PMID 8778584.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.