Pneumococcal conjugate vaccine
Pneumococcal conjugate vaccine is a pneumococcal vaccine and a conjugate vaccine used to protect infants, young children, and adults against disease caused by the bacterium Streptococcus pneumoniae (pneumococcus). It contains purified capsular polysaccharide of pneumococcal serotypes conjugated to a carrier protein to improve antibody response compared to the pneumococcal polysaccharide vaccine. The World Health Organization (WHO) recommends the use of the conjugate vaccine in routine immunizations given to children.[11]
 Prevenar 13 | |
| Vaccine description | |
|---|---|
| Target | Streptococcus pneumoniae |
| Vaccine type | Conjugate |
| Clinical data | |
| Trade names | Prevnar 20, Prevnar 13, Synflorix, others; discontinued Prevnar (PCV7) |
| Other names | PCV |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607021 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Intramuscular |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| ChemSpider |
|
| KEGG | |
Vaccine-mediated immunity is "conferred mainly by opsonophagocytic killing of S. pneumoniae."[12]
The most common side effects in children are decreased appetite, fever (only very common in children aged six weeks to five years), irritability, reactions at the site of injection (reddening or hardening of the skin, swelling, pain or tenderness), somnolence (sleepiness) and poor quality sleep.[13][12] In adults and the elderly, the most common side effects are decreased appetite, headaches, diarrhea, fever (only very common in adults aged 18 to 29 years), vomiting (only very common in adults aged 18 to 49 years), rash, reactions at the site of injection, limitation of arm movement, arthralgia and myalgia (joint and muscle pain), chills and fatigue.[13][12]
Brands
Pneumosil
Pneumosil is a decavalent pneumococcal conjugate vaccine produced by the Serum Institute of India. It contains the serotypes 1, 5, 6A, 6B, 7F, 9V, 14, 19A, 19F, and 23F, and was prequalified by WHO in January 2020.[14][15]
Prevnar

Prevnar 13 (PCV13) is produced by Pfizer (formerly Wyeth) and it replaced Prevnar, the pneumococcal 7-valent conjugate vaccine (PCV7).[16] It is a tridecavalent vaccine and thus contains thirteen serotypes of pneumococcus (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F).[17] These serotype sugars are conjugated to diphtheria carrier protein because this makes the antigenic and protective effect stronger (in most cases).[17][4] Prevnar 13 was approved for use in the European Union in December 2009.[13] In February 2010, Prevnar 13 was approved in the United States to replace Prevnar, the pneumococcal 7-valent conjugate vaccine (PCV7).[16][18] After waiting for the outcome of a trial underway in the Netherlands, the Centers for Disease Control and Prevention (CDC) recommended the vaccine for adults over age 65 in August 2014.[19]
Prevnar (PCV7) was a heptavalent vaccine, meaning that it contains the cell capsule sugars of seven serotypes of the bacteria S. pneumoniae (4, 6B, 9V, 14, 18C, 19F, and 23F) conjugated with diphtheria proteins.[20] It was manufactured by Wyeth (which was acquired by Pfizer).[21][22] Prevnar (PCV7) was approved for use in the United States in February 2000,[21][23] and vaccination with Prevnar was recommended for all children younger than two years and for unvaccinated children between 24 and 59 months old who were at high risk for pneumococcal infections.[24]
Prevnar was produced from the seven most prevalent strains of Streptococcus pneumoniae bacteria in the U.S. The bacterial capsule sugars, a characteristic of these pathogens, are linked (conjugated) to CRM197, a nontoxic recombinant variant of diphtheria toxin (from cultures of Corynebacterium diphtheriae).[12] This produces a more robust immune response (in most healthy persons). Further, aluminum is also added to the vaccine serum because it is an adjuvant, meaning it further enhances the immune response.[12]
The vaccine's polysaccharide sugars are grown separately in soy peptone broths.[12] Through reductive amination, the sugars are directly conjugated to the protein carrier CRM197 to form the glycoconjugate.[12] CRM197 is grown in C. diphtheriae strain C7 in a medium of casamino acids and yeast extracts.[25][12]
The Prevnar seven-valent formulation contained serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F, and resulted in a 98% probability of protection against these strains, which caused 80% of the pneumococcal disease in infants in the U.S. PCV7 is no longer produced.[26]
In 2010, Pfizer introduced Prevnar 13, which contains six additional strains (1, 3, 5, 6A, 19A and 7F), which protect against the majority of the remaining pneumococcal infections.[27]
In June 2021, the U.S. Food and Drug Administration (FDA) approved Prevnar 20, an icosavalent pneumococcal conjugate vaccine, which includes the serotypes 1, 3, 4, 5, 6A, 6B, 7F, 8, 9V, 10A, 11A, 12F, 14, 15B, 18C, 19A, 19F, 22F, 23F, and 33F for adults 18 years of age and older.[28][5] As of August 2022, seven serotypes remain experimental and were authorized only under "accelerated approval" by FDA, based on opsonophagocytic activity ("OPA") assay. Maintenance of this approval is ongoing and "may be contingent upon verification and description of clinical benefit in a confirmatory trial."[12]
In February 2022, the European Medicines Agency Approves Pfizer's Prevnar 20 (PVC20) under the brand name Apexxnar.[29][30]
Synflorix
Synflorix (PCV10) is produced by GlaxoSmithKline. It is a decavalent vaccine and thus contains ten serotypes of pneumococcus (1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, and 23F) which are conjugated to a carrier protein. Synflorix received a positive opinion from the European Medicines Agency (EMA) for use in the European Union in January 2009,[31] and GSK received European Commission authorization to market Synflorix in March 2009.[32][7]
Vaxneuvance
Vaxneuvance is a pneumococcal 15-valent conjugate vaccine created by Merck that was approved for medical use in the United States in July 2021.[6][33] The vaccine was developed under the code name "V114".[34] It is identical to PCV13, except that it adds serotypes 22F and 33F.[35] These two serotypes are particularly important because, after "widespread use of the PCV13…[vaccine] in many countries," these two serotypes are now "among leading serotypes causing IPD in children and adults."[35]
Vaxneuvance is indicated for the active immunization for the prevention of invasive disease caused by Streptococcus pneumoniae serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F and 33F in adults 18 years of age and older.[6][33][36]
On 14 October 2021, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Vaxneuvance, intended for prophylaxis against pneumococcal pneumonia and associated invasive disease.[37] The applicant for this medicinal product is Merck Sharp & Dohme B.V.[37] Vaxneuvance was approved for medical use in the European Union in December 2021.[8][9]
Schedule of vaccination
As with all immunizations, whether it is available or required, and under what circumstances, varies according to the decisions made by local public health agencies.
Children under the age of two years fail to mount an adequate response to the 23-valent adult vaccine, and so a pneumococcal conjugate vaccine is used. While this covers only seven strains out of more than ninety strains, these seven strains cause 80% to 90% of cases of severe pneumococcal disease, and it is considered to be nearly 100% effective against these strains.[38]
United Kingdom
The UK childhood vaccination schedule for infants born after 31 December 2019, consists of a primary course of one dose at twelve weeks of age with a second dose at one year of age.[39][40] For infants born before 1 January 2020 and those in Scotland, the childhood vaccination schedule consists of a primary course of two doses at eight and sixteen weeks of age with a final third dose at one year of age.[40]
Children at special risk (e.g., sickle cell disease and asplenia) require as full protection as can be achieved using the conjugated vaccine, with the more extensive polysaccharide vaccine given after the second year of life:[40]
| Age | 2–6 months | 7–11 months | 12–23 months |
| Conjugated vaccine | 3 × monthly dose | 2 × monthly dose | 2 doses, 2 months apart |
| Further dose in second year of life | |||
| 23-valent vaccine | Then after 2nd birthday single dose of 23-valent | ||
United States
In 2001, the Centers for Disease Control and Prevention (CDC), upon advice from its Advisory Committee on Immunization Practices (ACIP), recommended the vaccine be administered to every infant and young child in the United States. The resulting demand outstripped production, creating shortages not resolved until 2004. All children, according to the U.S. vaccination schedule, should receive four doses, at two months, four months, six months, and again between one year and fifteen months of age.[41][42]
The CDC updated the pneumococcal vaccine guidelines for adults 65 years of age or older in 2019.[43]
In October 2021, the CDC recommended that adults 65 years of age or older who have not previously received a pneumococcal conjugate vaccine or whose previous vaccination history is unknown should receive a pneumococcal conjugate vaccine (either PCV20 or PCV15).[44] If PCV15 is used, this should be followed by a dose of PPSV23.[44] The CDC recommended that adults aged 19 to 64 years with certain underlying medical conditions or other risk factors who have not previously received a pneumococcal conjugate vaccine or whose previous vaccination history is unknown should receive a pneumococcal conjugate vaccine (either PCV20 or PCV15).[44] If PCV15 is used, this should be followed by a dose of PPSV23.[44]
Efficacy
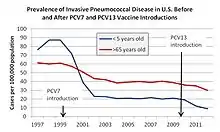
Prevnar-7 is designed to stop seven of about ninety pneumococcal serotypes which have the potential to cause invasive pneumococcal disease (IPD). In 2010, a 13-valent vaccine was introduced. Each year, IPD kills approximately one million children worldwide.[46] Since approval, Prevnar's efficacy in preventing IPD has been documented by a number of epidemiologic studies.[47][48][49] There is evidence that other people in the same household as a vaccinee also become relatively protected.[50] There is evidence that routine childhood vaccination reduces the burden of pneumococcal disease in adults and especially high-risk adults, such as those living with HIV/AIDS.[51]
The vaccine is, however, primarily developed for the U.S. and European epidemiological situation, and therefore it has only a limited coverage of serotypes causing serious pneumococcal infections in most developing countries.[52]
Evidence supporting addition to routine vaccination schedules
After introduction of the pneumococcal conjugate vaccine in 2000, several studies described a decrease in invasive pneumococcal disease in the United States. One year after its introduction, a group of investigators found a 69% drop in the rate of invasive disease in those of less than two years of age.[47] By 2004, all-cause pneumonia admission rates had declined by 39% (95% CI 22–52) and rates of hospitalizations for pneumococcal meningitis decreased by 66% (95% CI 56.3-73.5) in children younger than 2.[53][54]
Rates of invasive pneumococcal disease among adults have also declined since the introduction of the vaccine.[47][54]
Vaccination in low-income countries
Pneumococcal disease is the leading vaccine-preventable killer of young children worldwide, according to the World Health Organization (WHO). It killed more than 500,000 children younger than five years of age in 2008 alone.[55] Approximately ninety percent of these deaths occur in the developing world.[55] Historically 15–20 years pass before a new vaccine reaches one quarter of the population of the developing world.[56]
Pneumococcal vaccines Accelerated Development and Introduction Plan (PneumoADIP) was a GAVI Alliance (GAVI) funded project to accelerate the introduction of pneumococcal vaccinations into low-income countries through partnerships between countries, donors, academia, international organizations and industry. GAVI continues this work and as of March 2013, 25 GAVI-eligible and supported countries have introduced the pneumococcal conjugate vaccine. Further, 15 additional GAVI countries have plans to introduce the vaccine into their national immunization program and 23 additional countries have approved GAVI support to introduce the vaccine.[57]
Society and culture
Legal status
On 16 December 2021, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Apexxnar, intended for prophylaxis against pneumococcal pneumonia and associated invasive disease.[58] The applicant for this medicinal product is Pfizer Europe MA EEIG.[58] Apexxnar was approved for medical use in the European Union in February 2022.[10]
Research
Merck is investigating a 21-valent vaccine (code named V116) against pneumococcus serotypes.[61] The vaccine is geared towards persons living with HIV.[61]
References
- "Vaxneuvance APMDS". Therapeutic Goods Administration (TGA). 24 January 2022. Archived from the original on 5 February 2022. Retrieved 5 February 2022.
- "Summary Basis of Decision (SBD) for Vaxneuvance". Health Canada. 25 February 2022. Archived from the original on 29 May 2022. Retrieved 29 May 2022.
- "Summary Basis of Decision - Prevnar 20". Health Canada. 31 August 2022. Archived from the original on 29 September 2022. Retrieved 29 September 2022.
- "Prevnar 13- pneumococcal 13-valent conjugate vaccine injection, suspension". DailyMed. Archived from the original on 21 August 2021. Retrieved 20 August 2021.
- "Prevnar 20- pneumococcal 20-valent conjugate vaccine injection, suspension". DailyMed. Archived from the original on 21 August 2021. Retrieved 20 August 2021.
- "Vaxneuvance- pneumococcal 15-valent conjugate vaccine crm197 protein adsorbed injection, suspension". DailyMed. Archived from the original on 21 August 2021. Retrieved 20 August 2021.
- "Synflorix EPAR". European Medicines Agency (EMA). Archived from the original on 8 January 2021. Retrieved 13 July 2020.
- "Vaxneuvance EPAR". European Medicines Agency (EMA). Archived from the original on 18 January 2022. Retrieved 17 January 2022. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- "Vaxneuvance". Union Register of medicinal products. Archived from the original on 11 January 2022. Retrieved 11 January 2022.
- "Apexxnar EPAR". European Medicines Agency (EMA). 14 December 2021. Archived from the original on 3 March 2022. Retrieved 2 March 2022.
- World Health Organization (2019). "Pneumococcal conjugate vaccines in infants and children under 5 years of age: WHO position paper –February 2019". Wkly Epidemiol Rec. 94 (8): 85–104. hdl:10665/310970. Lay summary (PDF).
{{cite journal}}: Cite uses deprecated parameter|lay-url=(help) - "PREVNAR 20- pneumococcal 20-valent conjugate vaccine injection, suspension: Wyeth Pharmaceutical Division of Wyeth Holdings LLC". Philadelphia, PA 19101, US: Wyeth Pharmaceuticals, LLC (A subsidiary of Pfizer Inc.). June 2021. p. 11. Archived from the original on 5 September 2022. Retrieved 9 August 2022.
{{cite web}}: CS1 maint: date and year (link) CS1 maint: location (link) - "Prevenar 13 EPAR". European Medicines Agency (EMA). 26 March 2020. Archived from the original on 26 February 2021. Retrieved 26 March 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- "Gavi-supported pneumococcal conjugate vaccines profiles to support country decision making" (PDF). GAVI. 2019. Archived (PDF) from the original on 19 May 2020. Retrieved 8 April 2020.
- "Pneumosil, the new pneumococcal vaccine, achieves WHO prequalification, a key step toward improving access and affordability" (Press release). Serum Institute of India. PR Newswire. 28 January 2020. Archived from the original on 17 April 2021. Retrieved 8 April 2020.
- "FDA Approves Pneumococcal Disease Vaccine with Broader Protection" (Press release). 24 February 2010. Archived from the original on 11 September 2010. Retrieved 9 September 2010.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "Prevnar 13". U.S. Food and Drug Administration (FDA). 1 March 2018. STN 125324. Archived from the original on 27 November 2019. Retrieved 27 November 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "Prevnar 13". U.S. Food and Drug Administration (FDA). 12 March 2010. Archived from the original on 12 March 2010. Retrieved 27 November 2019.
{{cite web}}: CS1 maint: unfit URL (link) This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "Advisory Committee on Immunization Practices Votes to Recommend Pfizer's Prevnar 13 Vaccine in Adults Aged 65 Years and Older". MarketWatch.com. 13 August 2014. Archived from the original on 4 March 2016. Retrieved 27 April 2017.
- "Prevnar". U.S. Food and Drug Administration. 25 May 2022. Archived from the original on 1 September 2022. Retrieved 29 September 2022.
- "Prevnar". wayback.archive-it.org. 24 August 2009. Archived from the original on 22 July 2017. Retrieved 29 September 2022.
{{cite web}}: CS1 maint: bot: original URL status unknown (link) - "Pneumococcal 7-valent Conjugate Vaccine (Diphtheria CRM197 Protein)". Wyeth. 2006. Archived from the original on 15 June 2006.
- "February 17, 2000 Approval Letter". U.S. Food and Drug Administration (FDA). Archived from the original on 10 July 2009.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "American Academy of Pediatrics. Committee on Infectious Diseases. Policy statement: recommendations for the prevention of pneumococcal infections, including the use of pneumococcal conjugate vaccine (Prevnar), pneumococcal polysaccharide vaccine, and antibiotic prophylaxis". Pediatrics. 106 (2 Pt 1): 362–366. August 2000. doi:10.1542/peds.106.2.362. PMID 10920169.
- "Prevnar (Pneumococcal 7-valent Conjugate) drug description - prescription drugs and medications at RxList". Archived from the original on 11 December 2007. Retrieved 21 November 2007.
- "WHO SAGE evidence to recommendations table" (PDF). Archived (PDF) from the original on 9 May 2021. Retrieved 6 April 2020.
- Centers for Disease Control Prevention (CDC) (March 2010). "Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children - Advisory Committee on Immunization Practices (ACIP), 2010" (PDF). MMWR. Morbidity and Mortality Weekly Report. 59 (9): 258–261. PMID 20224542. Archived (PDF) from the original on 18 June 2022. Retrieved 29 September 2022.
- "Prevnar 20". U.S. Food and Drug Administration (FDA). 10 June 2021. Archived from the original on 10 June 2021. Retrieved 20 June 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "European Medicines Agency Approves Pfizer's 20-Valent Pneumococcal Conjugate Vaccine Against Invasive Pneumococcal Disease and Pneumonia in Adults (EU Apexxnar, U.S. Prevnar 20)". Pfizer (Press release). 15 February 2022. Archived from the original on 7 June 2022. Retrieved 9 June 2022.
- "European Medicines Agency info: Apexxnar". European Medicines Agency. 15 February 2022. Archived from the original on 3 March 2022. Retrieved 3 March 2022.
- "EMEA Document" (PDF). Emea.europa.eu. Archived from the original (PDF) on 19 February 2009. Retrieved 27 April 2017.
- "GSK Release". Gsk.com (Press release). Archived from the original on 4 August 2009. Retrieved 27 April 2017.
- "Vaxneuvance". U.S. Food and Drug Administration (FDA). 30 July 2021. Archived from the original on 21 August 2021. Retrieved 20 August 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "GRADE: 15-valent pneumococcal conjugate vaccine (PCV15) in series with 23-valent pneumococcal conjugate vaccine (PPSV23) for adults aged ≥65 years | CDC". www.cdc.gov. 27 January 2022. Archived from the original on 9 August 2022. Retrieved 9 August 2022.
- Stacey HL, Rosen J, Peterson JT, Williams-Diaz A, Gakhar V, Sterling TM, et al. (2019). "Safety and immunogenicity of 15-valent pneumococcal conjugate vaccine (PCV-15) compared to PCV-13 in healthy older adults". Human Vaccines & Immunotherapeutics. 15 (3): 530–539. doi:10.1080/21645515.2018.1532249. PMC 6605726. PMID 30648919.
- "Merck Announces U.S. FDA Approval of Vaxneuvance (Pneumococcal 15-valent Conjugate Vaccine) for the Prevention of Invasive Pneumococcal Disease in Adults 18 Years and Older Caused by 15 Serotypes" (Press release). Merck. 16 July 2021. Archived from the original on 21 August 2021. Retrieved 20 August 2021 – via Business Wire.
- "Vaxneuvance: Pending EC decision". European Medicines Agency (EMA). 13 October 2021. Archived from the original on 18 October 2021. Retrieved 15 October 2021. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- Childhood Pneumococcal Disease Archived 25 October 2006 at the Wayback Machine - information on the disease and the Prevnar vaccine, from the Victoria State (Australia) government. Includes possible side effects.
- "NHS vaccinations and when to have them". 31 July 2019. Archived from the original on 17 December 2021. Retrieved 3 September 2021.
- Ramsay M, ed. (January 2020). "Chapter 25: Pneumococcal". Immunisation against infectious disease. Public Health England. Archived from the original on 12 November 2019. Retrieved 3 September 2021.
- "Recommended Child and Adolescent Immunization Schedule for ages 18 years or younger, United States, 2019". Centers for Disease Control and Prevention (CDC). 5 February 2019. Archived from the original on 6 March 2016. Retrieved 3 November 2019.
- Nuorti JP, Whitney CG (December 2010). "Prevention of pneumococcal disease among infants and children - use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine - recommendations of the Advisory Committee on Immunization Practices (ACIP)" (PDF). MMWR. Recommendations and Reports. 59 (RR-11): 1–18. PMID 21150868. Archived (PDF) from the original on 2 September 2021. Retrieved 20 November 2021.
- Matanock A, Lee G, Gierke R, Kobayashi M, Leidner A, Pilishvili T (November 2019). "Use of 13-Valent Pneumococcal Conjugate Vaccine and 23-Valent Pneumococcal Polysaccharide Vaccine Among Adults Aged ≥65 Years: Updated Recommendations of the Advisory Committee on Immunization Practices" (PDF). MMWR. Morbidity and Mortality Weekly Report. 68 (46): 1069–1075. doi:10.15585/mmwr.mm6846a5. PMC 6871896. PMID 31751323. Archived (PDF) from the original on 20 November 2021. Retrieved 20 November 2021.
- "ACIP Vaccine Recommendations and Schedules". Centers for Disease Control and Prevention (CDC). Archived from the original on 20 November 2021. Retrieved 19 November 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "CDC - ABCs: Surveillance Reports main page - Active Bacterial Core surveillance". 19 July 2021. Archived from the original on 1 December 2020. Retrieved 10 September 2017.
- Allen A (21 June 2007). "What if a vaccine makes room for a new strain of a disease?". Slate.com. Archived from the original on 18 May 2011. Retrieved 27 April 2017.
- Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, et al. (May 2003). "Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine". The New England Journal of Medicine. 348 (18): 1737–1746. doi:10.1056/NEJMoa022823. PMID 12724479.
- Poehling KA, Talbot TR, Griffin MR, Craig AS, Whitney CG, Zell E, et al. (April 2006). "Invasive pneumococcal disease among infants before and after introduction of pneumococcal conjugate vaccine". JAMA. 295 (14): 1668–1674. doi:10.1001/jama.295.14.1668. PMID 16609088.
- Whitney CG, Pilishvili T, Farley MM, Schaffner W, Craig AS, Lynfield R, et al. (October 2006). "Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study". Lancet. 368 (9546): 1495–1502. doi:10.1016/S0140-6736(06)69637-2. PMID 17071283. S2CID 11834808. Archived from the original on 5 December 2020. Retrieved 5 July 2019.
- Millar EV, Watt JP, Bronsdon MA, Dallas J, Reid R, Santosham M, O'Brien KL (October 2008). "Indirect effect of 7-valent pneumococcal conjugate vaccine on pneumococcal colonization among unvaccinated household members". Clinical Infectious Diseases. 47 (8): 989–996. doi:10.1086/591966. PMID 18781875.
- Siemieniuk RA, Gregson DB, Gill MJ (November 2011). "The persisting burden of invasive pneumococcal disease in HIV patients: an observational cohort study". BMC Infectious Diseases. 11: 314. doi:10.1186/1471-2334-11-314. PMC 3226630. PMID 22078162.
- Barocchi MA, Censini S, Rappuoli R (April 2007). "Vaccines in the era of genomics: the pneumococcal challenge". Vaccine. 25 (16): 2963–2973. doi:10.1016/j.vaccine.2007.01.065. PMID 17324490.
- Grijalva CG, Nuorti JP, Arbogast PG, Martin SW, Edwards KM, Griffin MR (April 2007). "Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis". Lancet. 369 (9568): 1179–1186. doi:10.1016/S0140-6736(07)60564-9. PMID 17416262. S2CID 26494828.
- Tsai CJ, Griffin MR, Nuorti JP, Grijalva CG (June 2008). "Changing epidemiology of pneumococcal meningitis after the introduction of pneumococcal conjugate vaccine in the United States". Clinical Infectious Diseases. 46 (11): 1664–1672. doi:10.1086/587897. PMC 4822508. PMID 18433334.
- O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, et al. (September 2009). "Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates". Lancet. 374 (9693): 893–902. doi:10.1016/S0140-6736(09)61204-6. PMID 19748398. S2CID 18964449.
- "PneumoADIP - Need for PneumoADIP". Pneumoasdip.com. Archived from the original on 27 August 2021. Retrieved 27 April 2017.
- Johns Hopkins Bloomberg School of Public Health, International Vaccine Access Center (2013). "VIMS Report: Global vaccine introduction" (PDF). Jhsph.edu. Archived (PDF) from the original on 3 March 2016. Retrieved 27 April 2017.
- "Apexxnar: Pending EC decision". European Medicines Agency (EMA). 15 December 2021. Archived from the original on 17 December 2021. Retrieved 18 December 2021. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- "Pfizer Inc. 2020 Form 10-K Annual Report" (PDF). Pfizer. Archived (PDF) from the original on 23 March 2021. Retrieved 27 August 2021.
- Herper M (24 August 2020). "In the race for a Covid-19 vaccine, Pfizer turns to a scientist with a history of defying skeptics — and getting results". Stat News. Archived from the original on 21 December 2020. Retrieved 2 December 2020.
- "Safety and Immunogenicity of V116 in Adults Living With Human Immunodeficiency Virus (HIV) (V116-007, STRIDE-7)". ClinicalTrials.gov. 26 May 2022. Archived from the original on 9 August 2022. Retrieved 29 September 2022.
Further reading
- Centers for Disease Control and Prevention (2015). "Chapter 17: Pneumococcal Disease". In Hamborsky J, Kroger A, Wolfe S (eds.). Epidemiology and Prevention of Vaccine-Preventable Diseases (13th ed.). Washington, D.C.: Public Health Foundation.
External links
- "13-Valent Pneumococcal Vaccine". Drug Information Portal. U.S. National Library of Medicine.
- "Heptavalent pneumococcal conjugate vaccine". Drug Information Portal. U.S. National Library of Medicine.
- "Pneumococcal Conjugate (PCV13) Vaccine Information Statement". Centers for Disease Control and Prevention (CDC). 10 August 2021.