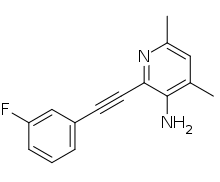
Raseglurant
Raseglurant (INN) (code name ADX-10059) is a negative allosteric modulator of the mGlu5 receptor and derivative of MPEP which was under development by Addex Therapeutics for the treatment of migraine, gastroesophageal reflux disease, and dental anxiety.[1][2][3] It reached phase II clinical trials for all of the aforementioned indications before being discontinued due to the observation of possible predictive signs of hepatotoxicity in patients with long-term use.[3][4][5]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C15H13FN2 |
| Molar mass | 240.281 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
See also
References
- Célanire S, Duvey G, Poli S, Rocher JP (January 2012). "mGluR2 activators and mGluR5 blockers advancing in the clinic for major CNS disorders.". Annual Reports in Medicinal Chemistry. Vol. 47. Academic Press. pp. 71–88. ISBN 978-0-12-397214-9.
- "Don't Dodge the Dentist – Tips for Dealing with Dental Anxiety". Retrieved 2017-04-05.
- Stein MB, Steckler T (30 July 2010). Behavioral Neurobiology of Anxiety and Its Treatment. Springer Science & Business Media. pp. 397–. ISBN 978-3-642-02912-7.
- Dominguez C (18 November 2010). Neurodegenerative Diseases. Springer Science & Business Media. pp. 120–. ISBN 978-3-642-16758-4.
- Shaheen NJ (25 March 2013). Benign and Neoplastic Conditions of the Esophagus, An Issue of Gastroenterology Clinics. Elsevier Health Sciences. pp. 119–. ISBN 978-1-4557-7175-2.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.