Thiamine
Thiamine, also known as thiamin and vitamin B1, is a vitamin, an essential micronutrient, which cannot be made in the body.[3][4] It is found in food and commercially synthesized to be a dietary supplement or medication.[1][5] Food sources of thiamine include whole grains, legumes, and some meats and fish.[1][6] Grain processing removes much of the thiamine content, so in many countries cereals and flours are enriched with thiamine.[1] Supplements and medications are available to treat and prevent thiamine deficiency and disorders that result from it, including beriberi and Wernicke encephalopathy. Other uses include the treatment of maple syrup urine disease and Leigh syndrome. They are typically taken by mouth, but may also be given by intravenous or intramuscular injection.[7]
 | |
 Skeletal formula and ball-and-stick model of the cation in thiamine | |
| Clinical data | |
|---|---|
| Pronunciation | /ˈθaɪ.əmɪn/ THY-ə-min |
| Other names | Vitamin B1, aneurine, thiamin |
| AHFS/Drugs.com | Monograph |
| License data | |
| Routes of administration | by mouth, IV, IM[1] |
| Drug class | vitamin |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 3.7% to 5.3% (Thiamine hydrochloride)[2] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEBI |
|
| ChEMBL |
|
| CompTox Dashboard (EPA) |
|
| Chemical and physical data | |
| Formula | C12H17N4OS+ |
| Molar mass | 265.36 g·mol−1 |
| 3D model (JSmol) |
|
SMILES
| |
InChI
| |
Thiamine supplements are generally well tolerated. Allergic reactions, including anaphylaxis, may occur when repeated doses are given by injection.[7][8] Thiamine is required for metabolism including that of glucose, amino acids, and lipids.[1] Thiamine is on the World Health Organization's List of Essential Medicines.[9] Thiamine is available as a generic medication, and in some countries as a non-prescription dietary supplement.[7]
Definition
Thiamine, also known as vitamin B1, is one of the B vitamins.[3][4] Unlike folate and vitamin B6, which occur in several chemically related forms known as vitamers, thiamine is only one chemical compound. It is soluble in water, methanol and glycerol, but practically insoluble in less polar organic solvents. Thiamine is usually supplied as a chloride salt. It is degraded by exposure to heat.[10][11] Within the body, the best-characterized form is thiamine pyrophosphate (TPP), also called thiamine diphosphate, a coenzyme in the catabolism of sugars and amino acids.[3]
Deficiency
Non-specific signs of thiamine deficiency include malaise, weight loss, irritability and confusion.[10][12] Well-known disorders caused by thiamine deficiency include beriberi, Wernicke–Korsakoff syndrome, optic neuropathy, Leigh's disease, African seasonal ataxia (or Nigerian seasonal ataxia), and central pontine myelinolysis.[13]
In Western countries, chronic alcoholism is a secondary cause. Also at risk are older adults, persons with HIV/AIDS or diabetes, and persons who have had bariatric surgery.[1] Varying degrees of thiamine deficiency have been associated with the long-term use of diuretics.[14][15]
Chemistry
Its structure consists of an aminopyrimidine and a thiazolium ring linked by a methylene bridge. The thiazole is substituted with methyl and hydroxyethyl side chains. Thiamine is a cation and is usually supplied as its chloride salt. The amino group can form additional salts with further acids. It is stable at acidic pH, but it is unstable in alkaline solutions and from exposure to heat.[10][11] Thiamine reacts strongly in Maillard-type reactions.[10] Oxidation yields the fluorescent derivative thiochrome.
Functions
Thiamine phosphate derivatives are involved in many cellular processes. The best-characterized form is thiamine pyrophosphate (TPP), a coenzyme in the catabolism of sugars and amino acids. Five natural thiamine phosphate derivatives are known: thiamine monophosphate (ThMP), thiamine diphosphate (ThDP), also called thiamine pyrophosphate (TPP), thiamine triphosphate (ThTP), adenosine thiamine triphosphate (AThTP) and adenosine thiamine diphosphate (AThDP). While the coenzyme role of thiamine diphosphate is well-known and extensively characterized, the non-coenzyme action of thiamine and derivatives may be realized through binding to a number of recently identified proteins which do not use the catalytic action of thiamine diphosphate.[16]
- Thiamine phosphate derivatives
 Thiamine monophosphate
Thiamine monophosphate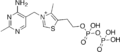 Thiamine diphosphate
Thiamine diphosphate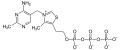 Thiamine triphosphate
Thiamine triphosphate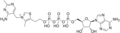 Adenosine thiamine triphosphate
Adenosine thiamine triphosphate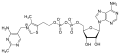 Adenosine thiamine diphosphate
Adenosine thiamine diphosphate
Thiamine diphosphate
No physiological role is known for the monophosphate. The diphosphate ThPP is physiologically relevant. Its synthesis is catalyzed by the enzyme thiamine diphosphokinase according to the reaction thiamine + ATP → ThDP + AMP (EC 2.7.6.2). ThDP is a coenzyme for several enzymes that catalyze the transfer of two-carbon units and in particular the dehydrogenation (decarboxylation and subsequent conjugation with coenzyme A) of 2-oxoacids (alpha-keto acids). Examples include:
- Present in most species
- pyruvate dehydrogenase and 2-oxoglutarate dehydrogenase (also called α-ketoglutarate dehydrogenase)
- branched-chain α-keto acid dehydrogenase
- 2-hydroxyphytanoyl-CoA lyase
- transketolase
- Present in some species:
- pyruvate decarboxylase (in yeast)
- several additional bacterial enzymes
The enzymes transketolase, pyruvate dehydrogenase (PDH), and 2-oxoglutarate dehydrogenase (OGDH) are all important in carbohydrate metabolism. The cytosolic enzyme transketolase is a key player in the pentose phosphate pathway, a major route for the biosynthesis of the pentose sugars deoxyribose and ribose. The mitochondrial PDH and OGDH are part of biochemical pathways that result in the generation of adenosine triphosphate (ATP), which is a major form of energy for the cell. PDH links glycolysis to the citric acid cycle, while the reaction catalyzed by OGDH is a rate-limiting step in the citric acid cycle. In the nervous system, PDH is also involved in the production of acetylcholine, a neurotransmitter, and for myelin synthesis.[11]
Thiamine triphosphate
ThTP was long considered a specific neuroactive form of thiamine, playing a role in chloride channels in the neurons of mammals and other animals, although this is not completely understood.[17] However, it was shown that ThTP exists in bacteria, fungi, plants and animals suggesting a much more general cellular role.[18] In particular in E. coli, it seems to play a role in response to amino acid starvation.[19]
Adenosine thiamine diphosphate
AThDP exists in small amounts in vertebrate liver, but its role remains unknown.[19]
Adenosine thiamine triphosphate
AThTP is present in Escherichia coli, where it accumulates as a result of carbon starvation. In E. coli, AThTP may account for up to 20% of total thiamine. It also exists in lesser amounts in yeast, roots of higher plants and animal tissue.[19]
Medical uses
Prenatal supplementation
Women who are pregnant or lactating require more thiamine due to thiamine being preferentially sent to the fetus and placenta, especially during the third trimester. For lactating women, thiamine is delivered in breast milk even if it results in thiamine deficiency in the mother.[4][20] Pregnant women with hyperemesis gravidarum are also at an increased risk for thiamine deficiency due to losses when vomiting.[21]
Thiamine is important for not only mitochondrial membrane development, but also synaptosomal membrane function.[22] It has also been suggested that thiamine deficiency plays a role in the poor development of the infant brain that can lead to sudden infant death syndrome (SIDS).[17]
Dietary recommendations
The US National Academy of Medicine updated the Estimated Average Requirements (EARs) and Recommended Dietary Allowances (RDAs) for thiamine in 1998. The EARs for thiamine for women and men aged 14 and over are 0.9 mg/day and 1.1 mg/day, respectively; the RDAs are 1.1 and 1.2 mg/day, respectively. RDAs are higher than EARs to provide adequate intake levels for individuals with higher than average requirements. The RDA during pregnancy and for lactating females is 1.4 mg/day. For infants up to the age of 12 months, the Adequate Intake (AI) is 0.2–0.3 mg/day and for children aged 1–13 years the RDA increases with age from 0.5 to 0.9 mg/day. As for safety, the IOM sets tolerable upper intake levels (ULs) for vitamins and minerals when evidence is sufficient. In the case of riboflavin there is no UL, as there is no human data for adverse effects from high doses. Collectively the EARs, RDAs, AIs and ULs are referred to as Dietary Reference Intakes (DRIs).[4]
The European Food Safety Authority (EFSA) refers to the collective set of information as Dietary Reference Values, with Population Reference Intakes (PRIs) instead of RDAs, and Average Requirements instead of EARs. AI and UL defined the same as in United States. For women (including those pregnant or lactating), men and children the PRI is 0.1 mg thiamine per megajoule (MJ) of energy consumed. As the conversion is 1 MJ = 239 kcal, an adult consuming 2390 kilocalories should be consuming 1.0 mg thiamine. This is slightly lower than the U.S. RDA.[23] The EFSA reviewed the same safety question and also reached the conclusion that there was not sufficient evidence to set a UL for thiamine.[24]
| United States | ||
| Age group | RDA (mg/day) | Tolerable upper intake level[4] |
|---|---|---|
| Infants 0–6 months | 0.2* | ND |
| Infants 6–12 months | 0.3* | |
| 1–3 years | 0.5 | |
| 4–8 years | 0.6 | |
| 9–13 years | 0.9 | |
| Females 14–18 years | 1.0 | |
| Males 14+ years | 1.2 | |
| Females 19+ years | 1.1 | |
| Pregnant/lactating females 14–50 | 1.4 | |
| * Adequate intake for infants, as an RDA has yet to be established[4] | ||
| European Food Safety Authority | ||
| Age group | Adequate Intake (mg/MJ)[24] | Tolerable upper limit[24] |
| All persons 7 months+ | 0.1 | ND |
Safety
Thiamine is generally well tolerated and non-toxic when administered orally.[7] Rarely, adverse side effects have been reported when thiamine is given intravenously including allergic reactions, nausea, lethargy, and impaired coordination.[24][3]
Labeling
For U.S. food and dietary supplement labeling purposes the amount in a serving is expressed as a percent of Daily Value (%DV). For thiamine labeling purposes 100% of the Daily Value was 1.5 mg, but as of May 27, 2016, it was revised to 1.2 mg to bring it into agreement with the RDA.[25][26] A table of the old and new adult daily values is provided at Reference Daily Intake.
Sources
Thiamine is found in a wide variety of processed and whole foods. Lentils, peas, whole grains, pork, and nuts are rich sources.[6][27]
To aid with adequate micronutrient intake, pregnant women are often advised to take a daily prenatal multivitamin. While micronutrient compositions vary among different vitamins, a typical daily prenatal vitamin product contains around 1.5 mg of thiamine.[28]
Antagonists
Thiamine in foods can be degraded in a variety of ways. Sulfites, which are added to foods usually as a preservative,[29] will attack thiamine at the methylene bridge in the structure, cleaving the pyrimidine ring from the thiazole ring.[12] The rate of this reaction is increased under acidic conditions. Thiamine is degraded by thermolabile thiaminases (present in raw fish and shellfish).[10] Some thiaminases are produced by bacteria. Bacterial thiaminases are cell surface enzymes that must dissociate from the membrane before being activated; the dissociation can occur in ruminants under acidotic conditions. Rumen bacteria also reduce sulfate to sulfite, therefore high dietary intakes of sulfate can have thiamine-antagonistic activities.
Plant thiamine antagonists are heat-stable and occur as both the ortho- and para-hydroxyphenols. Some examples of these antagonists are caffeic acid, chlorogenic acid, and tannic acid. These compounds interact with the thiamine to oxidize the thiazole ring, thus rendering it unable to be absorbed. Two flavonoids, quercetin and rutin, have also been implicated as thiamine antagonists.[12]
Food fortification
Some countries require or recommend fortification of grain foods such as wheat, rice or maize (corn) because processing lowers vitamin content.[30] As of February 2022, 59 countries, mostly in North and Sub-Saharan Africa, require food fortification of wheat, rice or maize with thiamine or thiamine mononitrate. The amounts stipulated range from 2.0 to 10.0 mg/kg.[31] An additional 18 countries have a voluntary fortification program. For example, the Indian government recommends 3.5 mg/kg for "maida" (white) and "atta" (whole wheat) flour.[32]
Synthesis
Biosynthesis
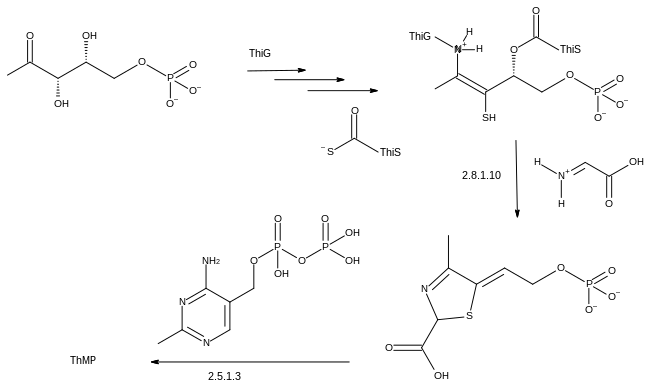
Thiamine biosynthesis occurs in bacteria, some protozoans, plants, and fungi.[33][34] The thiazole and pyrimidine moieties are biosynthesized separately and then combined to form thiamine monophosphate (ThMP) by the action of thiamine-phosphate synthase.
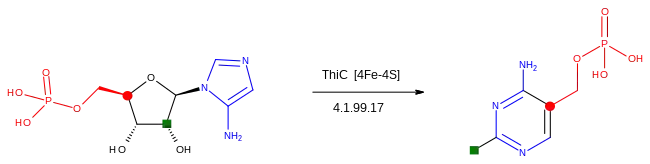
The pyrimidine ring system is formed in a reaction catalysed by phosphomethylpyrimidine synthase (ThiC), an enzyme in the radical SAM superfamily of iron–sulfur proteins, which use S-adenosyl methionine as a cofactor.[35][36]
The starting material is 5-aminoimidazole ribotide, which undergoes a rearrangement reaction via radical intermediates which incorporate the blue, green and red fragments shown into the product.[37][38]
The thiazole ring is formed in a reaction catalysed by thiazole synthase.[35] The ultimate precursors are 1-deoxy-D-xylulose 5-phosphate, 2-iminoacetate and a sulfur carrier protein called ThiS. These are assembled by the action of an additional protein component, ThiG.[39]
The final step to form ThMP involves decarboxylation of the thiazole intermediate, which reacts with the pyrophosphate derivative of the phosphomethylpyrimidine, itself a product of a kinase, phosphomethylpyrimidine kinase.[35]
The biosynthetic pathways may differ among organisms. In E. coli and other enterobacteriaceae, ThMP may be phosphorylated to the cofactor thiamine diphospate (ThDP) by a thiamine-phosphate kinase (ThMP + ATP → ThDP + ADP). In most bacteria and in eukaryotes, ThMP is hydrolyzed to thiamine, which may then be pyrophosphorylated to ThDP by thiamine diphosphokinase (thiamine + ATP → ThDP + AMP).
The biosynthetic pathways are regulated by riboswitches.[3] If there is sufficient thiamine present in the cell then the thiamine binds to the mRNAs for the enzymes that are required in the pathway and prevents their translation. If there is no thiamine present then there is no inhibition, and the enzymes required for the biosynthesis are produced. The specific riboswitch, the TPP riboswitch (or ThDP), is the only riboswitch identified in both eukaryotic and prokaryotic organisms.[40]
Laboratory synthesis
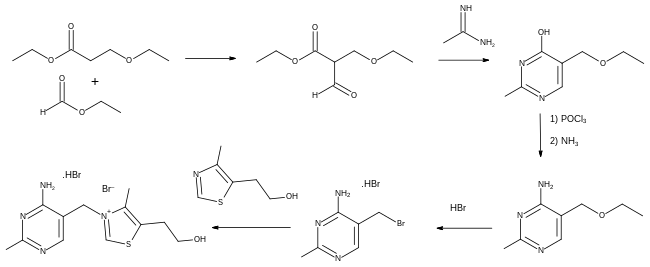
In the first total synthesis in 1936, ethyl 3-ethoxypropanoate was treated with ethyl formate to give an intermediate dicarbonyl compound which when reacted with acetamidine formed a substituted pyrimidine. Conversion of its hydroxyl group to an amino group was carried out by nucleophilic aromatic substitution, first to the chloride derivative using phosphorus oxychloride, followed by treatment with ammonia. The ethoxy group was then converted to a bromo derivative using hydrobromic acid, ready for the final stage in which thiamine (as its dibromide salt) was formed in an alkylation reaction using 4-methyl-5-(2-hydroxyethyl)thiazole.[41]: 7 [42]
Industrial synthesis
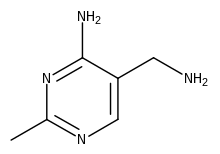
The 1936 laboratory-scale synthesis was developed by Merck & Co., allowing them to manufacture thiamine in Rahway in 1937.[42] However, an alternative route using the intermediate Grewe diamine (5-(aminomethyl)-2-methyl-4-pyrimidinamine), first published in 1937,[43] was investigated by Hoffman La Roche and competitive manufacturing processes followed. Efficient routes to the diamine have continued to be of interest.[42][44] In the European Economic Area, thiamine is registered under REACH regulation and between 100 and 1,000 tonnes per annum are manufactured or imported there.[45]
Absorption, metabolism and excretion
Thiamine phosphate esters in food are hydrolyzed to thiamine by intestinal alkaline phosphatase in the upper small intestine. At low concentrations, the absorption process is carrier-mediated. At higher concentrations, absorption also occurs via passive diffusion.[3] Active transport can be inhibited by alcohol consumption or by folate deficiency.[10]
The majority of thiamine in serum is bound to proteins, mainly albumin. Approximately 90% of total thiamine in blood is in erythrocytes. A specific binding protein called thiamine-binding protein (TBP) has been identified in rat serum and is believed to be a hormone-regulated carrier protein important for tissue distribution of thiamine.[12] Uptake of thiamine by cells of the blood and other tissues occurs via active transport and passive diffusion.[10] About 80% of intracellular thiamine is phosphorylated and most is bound to proteins. Two members of the SLC gene family of transporter proteins coded by the genes SLC19A2 and SLC19A3 are capable of the thiamine transport.[17] In some tissues, thiamine uptake and secretion appears to be mediated by a soluble thiamine transporter that is dependent on Na+ and a transcellular proton gradient.[12]
Human storage of thiamine is about 25 to 30 mg, with the greatest concentrations in skeletal muscle, heart, brain, liver, and kidneys. ThMP and free (unphosphorylated) thiamine is present in plasma, milk, cerebrospinal fluid, and, it is presumed, all extracellular fluid. Unlike the highly phosphorylated forms of thiamine, ThMP and free thiamine are capable of crossing cell membranes. Calcium and magnesium have been shown to affect the distribution of thiamine in the body and magnesium deficiency has been shown to aggravate thiamine deficiency.[17] Thiamine contents in human tissues are less than those of other species.[12][46]
Thiamine and its metabolites (2-methyl-4-amino-5-pyrimidine carboxylic acid, 4-methyl-thiazole-5-acetic acid, and others) are excreted principally in the urine.[3]
History
Thiamine was the first of the water-soluble vitamins to be isolated, in 1910.[47] Prior to that, observations in humans and in chickens had shown that diets of primarily polished white rice caused a disease "beriberi", but did not attribute it to the absence of a previously unknown essential nutrient.[48][49]
In 1884, Takaki Kanehiro, a surgeon general in the Japanese navy, rejected the previous germ theory for beriberi and hypothesized that the disease was due to insufficiencies in the diet instead.[48] Switching diets on a navy ship, he discovered that replacing a diet of white rice only with one also containing barley, meat, milk, bread, and vegetables, nearly eliminated beriberi on a nine-month sea voyage. However, Takaki had added many foods to the successful diet and he incorrectly attributed the benefit to increased protein intake, as vitamins were unknown substances at the time. The Navy was not convinced of the need for so expensive a program of dietary improvement, and many men continued to die of beriberi, even during the Russo-Japanese war of 1904–5. Not until 1905, after the anti-beriberi factor had been discovered in rice bran (removed by polishing into white rice) and in barley bran, was Takaki's experiment rewarded by making him a baron in the Japanese peerage system, after which he was affectionately called "Barley Baron".[48]
The specific connection to grain was made in 1897 by Christiaan Eijkman, a military doctor in the Dutch Indies, who discovered that fowl fed on a diet of cooked, polished rice developed paralysis, which could be reversed by discontinuing rice polishing.[49] He attributed beriberi to the high levels of starch in rice being toxic. He believed that the toxicity was countered in a compound present in the rice polishings.[50] An associate, Gerrit Grijns, correctly interpreted the connection between excessive consumption of polished rice and beriberi in 1901: He concluded that rice contains an essential nutrient in the outer layers of the grain that is removed by polishing.[51] Eijkman was eventually awarded the Nobel Prize in Physiology and Medicine in 1929, because his observations led to the discovery of vitamins.
In 1910, a Japanese agricultural chemist of Tokyo Imperial University, Umetaro Suzuki, first isolated a water-soluble thiamine compound from rice bran and named it as aberic acid. (He renamed it as Orizanin later.) He described the compound is not only anti beri-beri factor but also essential nutrition to human in the paper, however, this finding failed to gain publicity outside of Japan, because a claim that the compound is a new finding was omitted in translation from Japanese to German.[47] In 1911 a Polish biochemist Casimir Funk isolated the antineuritic substance from rice bran (the modern thiamine) that he called a "vitamine" (on account of its containing an amino group).[52][53] However, Funk did not completely characterize its chemical structure. Dutch chemists, Barend Coenraad Petrus Jansen and his closest collaborator Willem Frederik Donath, went on to isolate and crystallize the active agent in 1926,[54] whose structure was determined by Robert Runnels Williams, in 1934. Thiamine was named by the Williams team as "thio" or "sulfur-containing vitamin", with the term "vitamin" coming indirectly, by way of Funk, from the amine group of thiamine itself (by this time in 1936, vitamins were known to not always be amines, for example, vitamin C). Thiamine was synthesized in 1936 by the Williams group.[55]
Sir Rudolph Peters, in Oxford, introduced thiamine-deprived pigeons as a model for understanding how thiamine deficiency can lead to the pathological-physiological symptoms of beriberi. Indeed, feeding the pigeons upon polished rice leads to an easily recognizable behavior of head retraction, a condition called opisthotonos. If not treated, the animals died after a few days. Administration of thiamine at the stage of opisthotonos led to a complete cure within 30 minutes. As no morphological modifications were observed in the brain of the pigeons before and after treatment with thiamine, Peters introduced the concept of a biochemical lesion.[56] In 1937, when Lohman and Schuster showed that the diphosphorylated thiamine derivative (thiamine diphosphate, ThDP) was a cofactor required for the oxydative decarboxylation of pyruvate, the mechanism of action of thiamine in the cellular metabolism seemed to be elucidated.[57]
- Some contributors to the discovery of thiamine
 Takaki Kanehiro
Takaki Kanehiro Christiaan Eijkman
Christiaan Eijkman Gerrit Grijns
Gerrit Grijns Umetaro Suzuki
Umetaro Suzuki Casimir Funk
Casimir Funk Rudolph Peters
Rudolph Peters
Research
Benfotiamine, fursultiamine, sulbutiamine and others listed at Vitamin B1 analogues are synthetic derivatives of thiamine. Most were developed in Japan in the 1950s and 1960s as forms that were intended to improve absorption compared to thiamine.[58] Some are approved for use in some countries as a drug or non-prescription dietary supplement for treatment of diabetic neuropathy or other health conditions.[59][60][61]
See also
- Vitamin B1 analogues
References
- "Thiamin Fact Sheets for Health Professionals". Office of Dietary Supplements. 11 February 2016. Archived from the original on 30 December 2016. Retrieved 30 December 2016.
- Smithline HA, Donnino M, Greenblatt DJ (February 2012). "Pharmacokinetics of high-dose oral thiamine hydrochloride in healthy subjects". BMC Clinical Pharmacology. 12 (1): 4. doi:10.1186/1472-6904-12-4. PMC 3293077. PMID 22305197.
- Bettendorff L (2020). "Thiamine". In BP Marriott, DF Birt, VA Stallings, AA Yates (eds.). Present Knowledge in Nutrition, Eleventh Edition. London, United Kingdom: Academic Press (Elsevier). pp. 171–88. ISBN 978-0-323-66162-1.
- Institute of Medicine (1998). "Thiamin". Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: The National Academies Press. pp. 58–86. ISBN 978-0-309-06554-2. Archived from the original on 16 July 2015. Retrieved 29 August 2017.
- "Thiamine: MedlinePlus Drug Information". medlineplus.gov. Retrieved 30 April 2018.
- "Thiamin". Micronutrient Information Center, Linus Pauling Institute, Oregon State University. 2013. Retrieved 2 February 2022.
- American Society of Health-System Pharmacists. "Thiamine Hydrochloride". Drugsite Trust (Drugs.com). Retrieved 17 April 2018.
- Kliegman RM, Stanton B (2016). Nelson Textbook of Pediatrics. Elsevier Health Sciences. p. 322. ISBN 9781455775668.
There are no cases of adverse effects of excess thiamine... A few isolated cases of puritis...
- World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- Mahan LK, Escott-Stump S, eds. (2000). Krause's food, nutrition, & diet therapy (10th ed.). Philadelphia: W.B. Saunders Company. ISBN 978-0-7216-7904-4.
- Butterworth RF (2006). "Thiamin". In Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ (eds.). Modern Nutrition in Health and Disease (10th ed.). Baltimore: Lippincott Williams & Wilkins.
- Combs Jr GF (2008). The Vitamins: Fundamental Aspects in Nutrition and Health (3rd ed.). Ithaca, NY: Elsevier Academic Press. ISBN 978-0-12-183493-7.
- McCandless D (2010). Thiamine Deficiency and Associate Clinical Disorders. New York, NY: Humana Press. pp. 157–159. ISBN 978-1-60761-310-7.
- Katta N, Balla S, Alpert MA (July 2016). "Does Long-Term Furosemide Therapy Cause Thiamine Deficiency in Patients with Heart Failure? A Focused Review". The American Journal of Medicine. 129 (7): 753.e7–753.e11. doi:10.1016/j.amjmed.2016.01.037. PMID 26899752.
- Gomes F, Bergeron G, Bourassa MW, Fischer PR (August 2021). "Thiamine deficiency unrelated to alcohol consumption in high-income countries: a literature review". Annals of the New York Academy of Sciences. 1498 (1): 46–56. doi:10.1111/nyas.14569. PMC 8451800. PMID 33576090.
- Molecular mechanisms of the non-coenzyme action of thiamin in brain: biochemical, structural and pathway analysis : Scientific Reports Archived 31 July 2015 at the Wayback Machine
- Lonsdale D (March 2006). "A review of the biochemistry, metabolism and clinical benefits of thiamin(e) and its derivatives". Evidence-Based Complementary and Alternative Medicine. 3 (1): 49–59. doi:10.1093/ecam/nek009. PMC 1375232. PMID 16550223.
- Makarchikov AF, Lakaye B, Gulyai IE, Czerniecki J, Coumans B, Wins P, et al. (July 2003). "Thiamine triphosphate and thiamine triphosphatase activities: from bacteria to mammals". Cellular and Molecular Life Sciences. 60 (7): 1477–88. doi:10.1007/s00018-003-3098-4. PMID 12943234. S2CID 25400487.
- Bettendorff L (November 2021). "Update on Thiamine Triphosphorylated Derivatives and Metabolizing Enzymatic Complexes". Biomolecules. 11 (11): 1645. doi:10.3390/biom11111645. PMC 8615392. PMID 34827643.
- Butterworth RF (December 2001). "Maternal thiamine deficiency: still a problem in some world communities". The American Journal of Clinical Nutrition. 74 (6): 712–3. doi:10.1093/ajcn/74.6.712. PMID 11722950.
- Oudman E, Wijnia JW, Oey M, van Dam M, Painter RC, Postma A (May 2019). "Wernicke's encephalopathy in hyperemesis gravidarum: A systematic review". European Journal of Obstetrics, Gynecology, and Reproductive Biology. 236: 84–93. doi:10.1016/j.ejogrb.2019.03.006. hdl:1874/379566. PMID 30889425. S2CID 84184482.
- Kloss O, Eskin NA, Suh M (April 2018). "Thiamin deficiency on fetal brain development with and without prenatal alcohol exposure". Biochemistry and Cell Biology. 96 (2): 169–177. doi:10.1139/bcb-2017-0082. hdl:1807/87775. PMID 28915355.
- "Overview on Dietary Reference Values for the EU population as derived by the EFSA Panel on Dietetic Products, Nutrition and Allergies" (PDF). 2017. Archived (PDF) from the original on 28 August 2017.
- Tolerable Upper Intake Levels For Vitamins And Minerals (PDF), European Food Safety Authority, 2006, archived (PDF) from the original on 16 March 2016
- "Federal Register May 27, 2016 Food Labeling: Revision of the Nutrition and Supplement Facts Labels. FR page 33982" (PDF). Archived (PDF) from the original on 8 August 2016.
- "Daily Value Reference of the Dietary Supplement Label Database (DSLD)". Dietary Supplement Label Database (DSLD). Archived from the original on 7 April 2020. Retrieved 6 February 2022.
- "Thiamin content per 100 grams; select food subset, abridged list by food groups". United States Department of Agriculture, Agricultural Research Service, USDA Branded Food Products Database v.3.6.4.1. 17 January 2017. Archived from the original on 2 February 2017. Retrieved 27 January 2017.
- Kominiarek MA, Rajan P (November 2016). "Nutrition Recommendations in Pregnancy and Lactation". The Medical Clinics of North America. 100 (6): 1199–1215. doi:10.1016/j.mcna.2016.06.004. PMC 5104202. PMID 27745590.
- McGuire M, Beerman KA (2007). Nutritional Sciences: From Fundamentals to Foods. California: Thomas Wadsworth.
- "What nutrients are added to flour and rice in fortification?". Food Fortification Initiative. 2021. Retrieved 8 October 2021.
- "Map: Count of Nutrients In Fortification Standards". Global Fortification Data Exchange. Retrieved 11 October 2021.
- "Direction under Section 16(5) of Foods Safety and Standards Act, 2006 regarding Operationalisation of Food Safety & Standards (Fortification of Foods) Regulations, 2017 relating to standards for fortification of food" (PDF). Food Safety & Standards Authority of India (FSSAI). 19 May 2017. Retrieved 1 February 2022.
- Webb ME, Marquet A, Mendel RR, Rébeillé F, Smith AG (October 2007). "Elucidating biosynthetic pathways for vitamins and cofactors". Natural Product Reports. 24 (5): 988–1008. doi:10.1039/b703105j. PMID 17898894.
- Begley TP, Chatterjee A, Hanes JW, Hazra A, Ealick SE (April 2008). "Cofactor biosynthesis--still yielding fascinating new biological chemistry". Current Opinion in Chemical Biology. 12 (2): 118–25. doi:10.1016/j.cbpa.2008.02.006. PMC 2677635. PMID 18314013.
- R. Caspi (14 September 2011). "Pathway: superpathway of thiamine diphosphate biosynthesis I". MetaCyc Metabolic Pathway Database. Retrieved 1 February 2022.
- Holliday GL, Akiva E, Meng EC, Brown SD, Calhoun S, et al. (2018). "Atlas of the Radical SAM Superfamily: Divergent Evolution of Function Using a "Plug and Play" Domain". Radical SAM Enzymes. Methods in Enzymology. Vol. 606. pp. 1–71. doi:10.1016/bs.mie.2018.06.004. ISBN 9780128127940. PMC 6445391. PMID 30097089.
- Chatterjee A, Hazra AB, Abdelwahed S, Hilmey DG, Begley TP (November 2010). "A "radical dance" in thiamin biosynthesis: mechanistic analysis of the bacterial hydroxymethylpyrimidine phosphate synthase". Angewandte Chemie. 49 (46): 8653–8656. doi:10.1002/anie.201003419. PMC 3147014. PMID 20886485.
- Mehta AP, Abdelwahed SH, Fenwick MK, Hazra AB, Taga ME, Zhang Y, et al. (August 2015). "Anaerobic 5-Hydroxybenzimidazole Formation from Aminoimidazole Ribotide: An Unanticipated Intersection of Thiamin and Vitamin B₁₂ Biosynthesis". Journal of the American Chemical Society. 137 (33): 10444–10447. doi:10.1021/jacs.5b03576. PMC 4753784. PMID 26237670.
- Begley TP (February 2006). "Cofactor biosynthesis: an organic chemist's treasure trove". Natural Product Reports. 23 (1): 15–25. doi:10.1039/b207131m. PMID 16453030.
- Bocobza SE, Aharoni A (October 2008). "Switching the light on plant riboswitches". Trends in Plant Science. 13 (10): 526–33. doi:10.1016/j.tplants.2008.07.004. PMID 18778966.
- Tylicki A, Łotowski Z, Siemieniuk M, Ratkiewicz A (February 2018). "Thiamine and selected thiamine antivitamins - biological activity and methods of synthesis". Bioscience Reports. 38 (1). doi:10.1042/BSR20171148. PMC 6435462. PMID 29208764.
- Eggersdorfer M, Laudert D, Létinois U, McClymont T, Medlock J, Netscher T, Bonrath W (December 2012). "One hundred years of vitamins-a success story of the natural sciences". Angewandte Chemie. 51 (52): 12960–12990. doi:10.1002/anie.201205886. PMID 23208776.
- Todd AR, Bergel F (1937). "73. Aneurin. Part VII. A synthesis of aneurin". Journal of the Chemical Society (Resumed): 364. doi:10.1039/JR9370000364.
- Jiang M, Liu M, Huang H, Chen F (2021). "Fully Continuous Flow Synthesis of 5-(Aminomethyl)-2-methylpyrimidin-4-amine: A Key Intermediate of Vitamin B1". Organic Process Research & Development. 25 (10): 2331–2337. doi:10.1021/acs.oprd.1c00253. S2CID 242772232.
- "Substance Infocard". echa.europa.eu. Retrieved 11 May 2022.
- Bettendorff L, Mastrogiacomo F, Kish SJ, Grisar T (January 1996). "Thiamine, thiamine phosphates, and their metabolizing enzymes in human brain". Journal of Neurochemistry. 66 (1): 250–8. doi:10.1046/j.1471-4159.1996.66010250.x. PMID 8522961. S2CID 7161882.
- Suzuki U, Shimamura T (1911). "Active constituent of rice grits preventing bird polyneuritis". Tokyo Kagaku Kaishi. 32: 4–7, 144–146, 335–358. doi:10.1246/nikkashi1880.32.4.
- McCollum EV. A History of Nutrition. Cambridge, Massachusetts: Riverside Press, Houghton Mifflin (1957).
- Eijkman C (1897). "Eine Beriberiähnliche Krankheit der Hühner" [A disease of chickens which is similar to beriberi]. Archiv für Pathologische Anatomie und Physiologie und für Klinische Medicin. 148 (3): 523–32. doi:10.1007/BF01937576. S2CID 38445999.
- "The Nobel Prize and the Discovery of Vitamins". nobelprize.org.
- Grijns G (1901). "Over polyneuritis gallinarum" [On polyneuritis gallinarum]. Geneeskundig Tijdschrift voor Nederlandsch-Indië (Medical Journal for the Dutch East Indies). 41 (1): 3–11.
- Funk C (December 1911). "On the chemical nature of the substance which cures polyneuritis in birds induced by a diet of polished rice". The Journal of Physiology. 43 (5): 395–400. doi:10.1113/jphysiol.1911.sp001481. PMC 1512869. PMID 16993097.
- Funk C (1912). "The etiology of the deficiency diseases. Beri-beri, polyneuritis in birds, epidemic dropsy, scurvy, experimental scurvy in animals, infantile scurvy, ship beri-beri, pellagra". Journal of State Medicine. 20: 341–368. The word "vitamine" is coined on p. 342: "It is now known that all these diseases, with the exception of pellagra, can be prevented and cured by the addition of certain preventative substances; the deficient substances, which are of the nature of organic bases, we will call "vitamines"; and we will speak of a beri-beri or scurvy vitamine, which means a substance preventing the special disease."
- Jansen BC, Donath WF (1926). "On the isolation of antiberiberi vitamin". Proc. Kon. Ned. Akad. Wet. 29: 1390–1400.
- Williams RR, Cline JK (1936). "Synthesis of Vitamin B1". Journal of the American Chemical Society. 58 (8): 1504–05. doi:10.1021/ja01299a505.
- Peters RA (1936). "The biochemical lesion in vitamin B1 deficiency. Application of modern biochemical analysis in its diagnosis". Lancet. 230 (5882): 1161–1164. doi:10.1016/S0140-6736(01)28025-8.
- Lohmann K, Schuster P (1937). "Untersuchungen über die Cocarboxylase". Biochem. Z. 294: 188–214.
- Bettendorff L (2014). "Chapter 7 - Thiamine". In Zempleni J, Suttie JW, Gregory JF, Stover PJ (eds.). Handbook of vitamins (Fifth ed.). Hoboken: CRC Press. pp. 267–324. ISBN 9781466515574.
- Zaheer A, Zaheer F, Saeed H, Tahir Z, Tahir MW (April 2021). "A Review of Alternative Treatment Options in Diabetic Polyneuropathy". Cureus. 13 (4): e14600. doi:10.7759/cureus.14600. PMC 8139599. PMID 34040901.
- McCarty MF, Inoguchi T (2008). "11. Targeting Oxidant Stress as a Strategy for Preventing Vascular Complications of Diabetes and Metabolic Syndrome". In Pasupuleti VK, Anderson JW (eds.). Nutraceuticals, glycemic health and type 2 diabetes (1st ed.). Ames, Iowa: Wiley-Blackwell/IFT Press. p. 213. ISBN 9780813804286.
- Lonsdale D (September 2004). "Thiamine tetrahydrofurfuryl disulfide: a little known therapeutic agent". Medical Science Monitor. 10 (9): RA199–203. PMID 15328496.
External links
- "Thiamine". Drug Information Portal. U.S. National Library of Medicine.