Benzylpiperazine
Benzylpiperazine (BZP) is a recreational drug with euphoriant and stimulant properties. The effects produced by BZP are comparable to those produced by amphetamine.[4] Adverse effects have been reported following its use including acute psychosis, renal toxicity and seizures.[5] Deaths from piperazine derivatives are extremely rare, but there has been at least one death apparently due to BZP alone.[6] Its sale is banned in several countries, including Australia, Canada, New Zealand, the United States, the Republic of Ireland, the United Kingdom, Bulgaria, Romania and other parts of Europe.[7][8]
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral, intravenous, insufflation |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Unknown |
| Metabolism | Hepatic |
| Elimination half-life | 5.5 Hours[3] |
| Excretion | Renal |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.018.567 |
| Chemical and physical data | |
| Formula | C11H16N2 |
| Molar mass | 176.263 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
History
Development history
BZP was first synthesized by Burroughs Wellcome & Company in 1944.[9] It is often claimed that it was originally synthesized as a potential antihelminthic (anti-parasitic) agent for use in farm animals,[10] but its synthesis is thought to pre-date their interest in piperazines as anthelmintics.[9] Even so, the majority of the early work with the piperazines were investigations into their potential use as anthelmintics, with the earliest clinical trials in the literature relating to piperazine being articles in the British Medical Journal in the 1950s.[11][12] It was discovered that BZP had side effects and was largely abandoned as a worm treatment. It next appears in the literature in the 1970s when it was investigated as a potential antidepressant medication,[9] but rejected when research reported that BZP had amphetamine-like effects and was liable to abuse. The study suggested that BZP "should be placed under statutory control similar to those regulating the use of amphetamine".[13]
Recreational history
In 1996, the United States Drug Enforcement Administration noted that it was being recreationally used in California.[9] It also reported that BZP was being used as an adulterant in illicit drugs. In around 2000, its use increased worldwide,[9] which was soon followed by legislative control in Europe and the United States. In New Zealand, it was initially legal, but was restricted in 2005 and later reclassified as Class C due to new evidence in 2008.[9] It was widely used before its reclassification, and an estimated 5 million pills were sold in New Zealand in 2007.[14] BZP, which is often mixed with TFMPP, has been claimed to be a safer alternative to other illicit street drugs.[9] It is also used as an adulterant of or substitute for MDMA,[15] which has similar effects, and is sometimes marketed as "ecstasy", a colloquial term for MDMA.[9]
BZP has also been used and prohibited in horse racing and athletics.[9]
Production and distribution

BZP is a piperazine derivative which comes as either the hydrochloride salt or a free base. The hydrochloride salt is a white solid while the base form is a slightly yellowish-green liquid. BZP base is corrosive and causes burns.[16]
BZP is often marketed ostensibly as a "dietary supplement" to avoid meeting stricter laws that apply to medicines and drugs, despite the fact that BZP has no dietary value. As of late 2005, the Misuse of Drugs Act ensured it can no longer be classified or marketed as a dietary supplement in New Zealand.[17] Some retailers claim that BZP is a "natural" product, describing it as a "pepper extract" or "herbal high," when in fact the drug is entirely synthetic,[14] and has not been found to occur naturally.[18]
Pharmacodynamics
BZP has been shown to have a mixed mechanism of action, acting on the serotonergic and dopaminergic receptor systems in a similar fashion to MDMA.[19] BZP has amphetamine-like actions on the serotonin reuptake transporter, which increase serotonin concentrations in the extracellular fluids surrounding the cell and thereby increasing activation of the surrounding serotonin receptors.[20] BZP has a lower potency effect on the noradrenaline reuptake transporter and the dopamine reuptake transporter.[19] BZP has a high affinity action at the alpha2-adrenoreceptor, it is an antagonist at the receptor, like yohimbine, which inhibits negative feedback, causing an increase in released noradrenaline.[21] Another study[22] lists 1-Benzylpiperazine (BZP)'s Release DAT, NET, and SERT EC50s (i.e. a measure of potency for the release of neurotransmitters via BZP's affinity for the dopamine, norepinephrine, and serotonin transporters respectively--whereby those transporter are induced to shuttle neurotransmitters out of neurons and deposit them in the synaptic gap) as 175, 62, and 6050; for comparison, the values listed for d-amphetamine (25, 7, and 1765) and d-methamphetamine (25, 12, and 736) show a similar DAT:NET affinity ratio as well as minor SERT activity which suggests BZP possesses similar activity to the two aforementioned drugs (when dosed ~7x higher due to lower potency) rather than serotonergic substituted amphetamines like MDMA.
BZP also acts as a non-selective serotonin receptor agonist on a wide variety of serotonin receptors.[20] Binding to 5HT2A receptors may explain its mild hallucinogenic effects at high doses, while partial agonist or antagonist effects at the 5HT2B receptors may explain some of BZPs peripheral side effects, as this receptor is expressed very densely in the gut, and binding to 5HT3 receptors may explain the common side effect of headaches, as this receptor is known to be involved in the development of migraine headaches.[23]: 652
Effects

The effects of BZP are largely similar to amphetamines,[5][24] with one study finding that former amphetamine addicts were unable to distinguish between dextroamphetamine and BZP administered intravenously.[13] A 2005 study has shown that mixtures of BZP with other piperazine drugs such as TFMPP share certain pharmacodynamic traits with MDMA.[25]
Subjective effects
Upon ingestion of between 50 mg and 200 mg of BZP, the user may experience any or all of the following:
- Feelings of euphoria, wonder, amazement, well-being, energy and elation
- Rapid mood elevation
- Enhanced sociability
- Enhanced appreciation of music
- Increased desire to move, also slight increase in stereotypy
- Skin tingling
- Decreased appetite
- Repetitive thought patterns
- Actual and perceived changes in body temperature
- Mild jaw clenching/bruxism
- Increased heart rate
- Dilation of pupils (see photo)
- Nausea
- Flushing
- Mild xerostomia (dry mouth)
- Slight urinary incontinence, often described as "leaking" a small amount of urine after urinating (not due to loss of bladder control)
Later Effects:[26]
- Mild headache
- Nausea
- Hangover-like symptoms (common with high doses)
- Fatigue
- Indigestion (similar to acid indigestion/heartburn)
- Increased hunger (and sometimes thirst)
- Insomnia
- Confusion
- Depression (particularly with frequent/heavy use)
Tolerance
Research into BZP's tolerance is sparse.[6] Anecdotal evidence from online sources claim tolerance to the central action of BZP will develop quickly.[21] Due to tiredness associated with the body's recovery from stimulants, such as BZP, it is uncommon for users to be able to sustain a week-long intake.[23]: 653
Toxic effects

As with most sympathomimetic stimulants, significant side effects are associated with BZP use. It reportedly produces insomnia and a mild to severe hangover after the drug effect wears off.[26] The majority of the toxic effects information came from a 2005 study that recorded all presentations associated with party pill use at the Emergency Department of Christchurch Hospital, New Zealand. Fourteen toxic seizures were recorded in two patients with life-threatening toxicity with status epilepticus and severe respiratory and metabolic acidosis.[28] The results of this study and others like it showed that BZP can cause unpredictable and serious toxicity in some individuals,[26][29] but the data and dosage collection were reliant on self reporting by drug users, which may result in under-reporting (or over-reporting), and there were complicating factors like the frequent presence of alcohol and other drugs.[29]
The major side effects include dilated pupils, blurred vision, dryness of the mouth, extreme alertness, pruritus, confusion, agitation, tremor, extrapyramidal symptoms (dystonia, akathisia), headache, dizziness, anxiety, insomnia, vomiting, chest pain, hallucinations, paresthesia, tachycardia, hypertension, palpitations, collapse, hyperventilation, sweating, hyperthermia and problems with urine retention.[5][26][30][28][29] The more severe toxic effects include psychosis or adverse psychiatric events,[31][32] renal toxicity,[18] respiratory failure,[5] hyperthermia,[5] serotonin syndrome,[5] rhabdomyolysis[33] and seizure.[26][28] Blood benzylpiperazine concentrations have been measured either to confirm clinical intoxication or as part of a medicolegal death investigation.[34]
Risk of fatality
Ingestion of piperazine derivatives alone rarely causes death.[6] A retrospective study carried out at an Auckland emergency department found that BZP presentations only made a minor contribution to their overdose database, with most cases not producing any significant toxicity.[29] One death has been attributed to ingestion of BZP alone; in this case its blood concentration was measured to be 8 mg/L.[6]
Combined with alcohol or other illicit drugs, such as TFMPP and MDMA, multiple deaths have been reported.[6] A combination of BZP and MDMA ingested by a 23-year-old DJ nearly resulted in death. He was put into an induced coma, and later recovered.[35] In another case in Zurich in 2001, a 23-year-old who had taken BZP and MDMA died from a massive cerebral edema 57 hours after hospital admission.[36]
Addictive effects
BZP has not been found to be physically addictive in humans.[9] Most users of BZP say they could stop, but do not want to.[37] Studies undertaken on animals have indicated that BZP can substitute for methamphetamine in addicted rats, although it is one-tenth as potent and produces correspondingly weaker addictive effects.[38]
Legal status
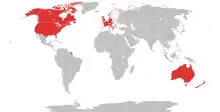
Red: Prohibited
Pink: European Union, requested prohibition
Gray: Unknown
BZP is banned in Australia, Austria, Canada, Denmark, Estonia, France, Germany, Greece, Ireland, Italy, Japan, Malta, Poland, and Sweden.[14][39] BZP is not controlled under any UN convention, so the compounds themselves are legal throughout most of the world, although in most countries their use is restricted to pharmaceutical manufacturing and recreational use is unknown.[39]
Australia
BZP is banned in all Australian states. Victoria, the last state in which it was legal, changed its classification on 1 September 2006,[40] when BZP and piperazine analogs become illegal in the federal schedules, which are enacted by all Australian states and territories.
Canada
In Canada, Benzylpiperazine and salts of benzylpiperazine are classified as Schedule III controlled substances under the Controlled Drugs and Substances Act.[41]
European Union
Benzylpiperazine was the subject of a European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) risk assessment to determine to determine how to control it throughout the European Union. The risk assessment came about as the result of a joint Europol – EMCDDA report which concluded that BZP needs to be looked at in more detail. The report was published in June 2007,[42] and concluded that the use of BZP can lead to medical problems even if the long effects are still unknown. Taking this concession as a basis, the European Commission asked the Council to place BZP under control of the UN Convention on Psychotropic Substances.[43] On 4 March 2008, the EU requested countries to place BZP under control within a year.[44]
New Zealand
Based on the recommendation of the EACD, the New Zealand government passed legislation which placed BZP, along with other piperazine derivatives (TFMPP, mCPP, pFPP, MeOPP, and MBZP), into Class C of the Misuse of Drugs Act 1975. A ban was intended to come into effect in New Zealand on 18 December 2007, but the law change did not go through until the following year, and the sale of BZP and the other listed piperazines became illegal in New Zealand as of 1 April 2008. An amnesty for possession and usage of these drugs was in effect until October 2008, at which point they became completely illegal.[45]
United Kingdom
Piperazine and salts of piperazine are classified as Prescription Only Medicines in the UK. Any products containing salts of piperazine would be licensable under the Medicines Act[46] and consequently anyone manufacturing and supplying it legally must hold the relevant licenses to do so. BZP is not a salt of piperazine, but mislabelling of BZP products as containing "piperazine blend" resulted in some prosecutions of suppliers in the UK by the Medicines and Healthcare Products Regulatory Agency, although none were successful.[47] In May 2009, the Home Office announced plans to ban BZP,[48] and launched a consultation on the proposal.[49] In October 2009, it was announced that from 23 December 2009, BZP and related piperazines would be Class C drugs under the Misuse of Drugs Act.[50]
United States
The drug was federally classified as a Schedule I controlled substance in the United States in 2002,[16] following a report by the DEA which incorrectly stated that BZP was 10 to 20 times more potent than amphetamine,[51] when in fact BZP is ten times less potent than dexamphetamine.[52] It is also illegal at the state level in Florida, Oklahoma, Vermont, and Virginia.[39]
Chemical derivatives
- Pharmaceuticals
- Befuraline – Antidepressant
- Bifeprunox – Antipsychotic
- Buclizine – Antihistamine
- Chlorbenzoxamine – Gastrointestinal agent
- Fipexide – Nootropic
- Imatinib – Anticancer agent
- Meclozine – Antihistamine
- Piberaline – Antidepressant
- Piribedil – Antiparkinsonian agent
- Trimetazidine – Antianginal
- Vesnarinone – Cardiotonic
- Designer drugs
- 3-Methylbenzylpiperazine
- 4-Methyl-1-benzylpiperazine (MBZP)
- 4-Bromo-2,5-dimethoxy-1-benzylpiperazine (2C-B-BZP)
- 1,4-Dibenzylpiperazine (DBZP)
- 3,4-Methylenedioxy-1-benzylpiperazine (MDBZP)
Diphenylmethylpiperazines are also similar to benzylpiperazines.
See also
- Diphenylmethylpiperazine
- Phenylpiperazine
- Pyrimidinylpiperazine
References
- "Controlled Drugs and Substances Act : Legislative history · Schedule III · Sections 2 to 32: Methylphenidate to BZP and TFMPP. Isomer Design". Retrieved 24 November 2012.
- "Amending Schedule III to the Controlled Drugs and Substances Act (BZP and TFMPP)". Canada Gazette. Retrieved 24 November 2012.
- Antia U, Lee HS, Kydd RR, Tingle MD, Russell BR (April 2009). "Pharmacokinetics of 'party pill' drug N-benzylpiperazine (BZP) in healthy human participants". Forensic Sci. Int. 186 (1–3): 63–7. doi:10.1016/j.forsciint.2009.01.015. PMID 19261399.
- Johnstone, Alice C.; Lea, Rod A.; Brennan, Katie A.; Schenk, Susan; Kennedy, Martin A.; Fitzmaurice, Paul S. (November 2007). "Review: Benzylpiperazine: a drug of abuse?". Journal of Psychopharmacology. 21 (8): 888–894. doi:10.1177/0269881107077260. ISSN 0269-8811. PMID 17606471. S2CID 27130853.
- Schep LJ, Slaughter RJ, Vale JA, Beasley DM, Gee P (March 2011). "The clinical toxicology of the designer "party pills" benzylpiperazine and trifluoromethylphenylpiperazine". Clin Toxicol. 49 (3): 131–41. doi:10.3109/15563650.2011.572076. PMID 21495881. S2CID 42491343.
- Gee, Paul; Schep, Leo J. (2022). "Chapter 12: 1-Benzylpiperazine and other piperazine-based stimulants". In Dargan, Paul; Wood, David (eds.). Novel Psychoactive Substances (2nd ed.). Elsevier. pp. 301–332. doi:10.1016/b978-0-12-818788-3.00009-7. ISBN 978-0-12-818788-3. S2CID 239180001.
- Topping, Alexandra (18 June 2007). "Legal dance drug faces ban amid fears over side-effects". The Guardian. Retrieved 26 May 2008.
- Examiner (18 June 2007). "Harney announces ban on stimulant BZP". The Irish Examiner. Archived from the original on 6 April 2009. Retrieved 28 December 2009.
- Kerr, J. R.; Davis, L. S. (March 2011). "Benzylpiperazine in New Zealand: brief history and current implications". Journal of the Royal Society of New Zealand. 41 (1): 155–164. doi:10.1080/03036758.2011.557036. ISSN 0303-6758.
- "Lay off the party pills". New Zealand Medical Association. 1 November 2006. Retrieved 22 April 2007.
- White R, Standen O (1953). "Piperazine in the Treatment of Threadworms in Children". British Medical Journal. 2 (4839): 755–7. doi:10.1136/bmj.2.4839.755. PMC 2029560. PMID 13082101.
- Standen O (1955). "Activity of Piperazine, in vitro, Against Ascaris lumbricoides". British Medical Journal. 2 (4930): 20–2. doi:10.1136/bmj.2.4930.20-a. PMC 1980175. PMID 14378628.
- Campbell H, Cline W, Evans M, Lloyd J, Peck A (1973). "Comparison of the effects of dexamphetamine and 1-benzylpiperazine in former addicts". Eur J Clin Pharmacol. 6 (3): 170–6. doi:10.1007/BF00558281. PMID 4586849. S2CID 5648863.
- Gee P, Fountain J (2007). "Party on? BZP party pills in New Zealand" (PDF). N Z Med J. 120 (1249): U2422. PMID 17308559. Archived from the original (PDF) on 13 October 2007.
- "DEA Microgram December 2008: Ecstasy Mimic Tablets". 25 December 2008. Archived from the original on 14 January 2009.
- "Drugs and Chemicals of Concern: N-Benzylpiperazine". US DEA. June 2006. Archived from the original on 7 April 2007. Retrieved 22 April 2007.
- "Misuse of Drugs Amendment Act 2005" (PDF). New Zealand Government. 17 June 2005. Archived from the original (PDF) on 16 July 2007. Retrieved 22 April 2007.
- Alansari M, Hamilton D (2006). "Nephrotoxicity of BZP-based herbal party pills: a New Zealand case report". N Z Med J. 119 (1233): U1959. PMID 16680176.
- Baumann M, Clark R, Budzynski A, Partilla J, Blough B, Rothman R (2004). "Effects of "Legal X" piperazine analogs on dopamine and serotonin release in rat brain". Ann N Y Acad Sci. 1025 (1): 189–97. Bibcode:2004NYASA1025..189B. doi:10.1196/annals.1316.024. PMID 15542717. S2CID 9195383.
- Tekes K, Tóthfalusi L, Malomvölgyi B, Hermán F, Magyar K (1987). "Studies on the biochemical mode of action of EGYT-475, a new antidepressant". Pol J Pharmacol Pharm. 39 (2): 203–11. PMID 2448760.
- BilZ0r (November 2003). "Neuropharmacology of BZP". Erowid.org. Retrieved 22 April 2007.
- Rothman, Richard B; Baumann, Michael H (2006). "Therapeutic potential of monoamine transporter substrates". Curr Top Med Chem. 6 (17): 1845–1859. doi:10.2174/156802606778249766. PMID 17017961. Retrieved 16 July 2022.
- Nikolova, I.; Danchev, N. (2008). "Piperazine Based Substances of Abuse: A new Party Pills on Bulgarian Drug Market". Biotechnology & Biotechnological Equipment. 22 (2): 652–655. doi:10.1080/13102818.2008.10817529. ISSN 1310-2818. S2CID 84913504.
- Fantegrossi W, Winger G, Woods J, Woolverton W, Coop A (2005). "Reinforcing and discriminative stimulus effects of 1-benzylpiperazine and trifluoromethylphenylpiperazine in rhesus monkeys". Drug Alcohol Depend. 77 (2): 161–8. doi:10.1016/j.drugalcdep.2004.07.014. PMID 15664717.
- Baumann M, Clark R, Budzynski A, Partilla J, Blough B, Rothman R (2005). "N-substituted piperazines abused by humans mimic the molecular mechanism of 3,4-methylenedioxymethamphetamine (MDMA, or 'Ecstasy')". Neuropsychopharmacology. 30 (3): 550–60. doi:10.1038/sj.npp.1300585. PMID 15496938. S2CID 24217984.
- Nicholson T (2006). "Prevalence of use, epidemiology and toxicity of 'herbal party pills' among those presenting to the emergency department". Emergency Medicine Australasia. 18 (2): 180–4. doi:10.1111/j.1742-6723.2006.00826.x. PMID 16669944. S2CID 26016419.
- "Erowid experience vault: Lazer Light Loving BZP & TFMPP". Erowid. 28 October 2004. Retrieved 26 May 2008.
- Gee P, Richardson S, Woltersdorf W, Moore G (2005). "Toxic effects of BZP-based herbal party pills in humans: a prospective study in Christchurch, New Zealand" (PDF). N Z Med J. 118 (1227): U1784. PMID 16372033. Archived from the original (PDF) on 7 October 2008.
- Theron L, Jansen K, Miles J (2007). "Benzylpiperizine-based party pills' impact on the Auckland City Hospital Emergency Department Overdose Database (2002–2004) compared with ecstasy (MDMA or methylene dioxymethamphetamine), gamma hydroxybutyrate (GHB), amphetamines, cocaine and alcohol" (PDF). N Z Med J. 120 (1249): U2416. PMID 17308553. Archived from the original (PDF) on 12 October 2007.
- Gee P, Gilbert M, Richardson S, Moore G, Paterson S, Graham P (November 2008). "Toxicity from the recreational use of 1-benzylpiperazine". Clin Toxicol. 46 (9): 802–7. doi:10.1080/15563650802307602. PMID 18821145. S2CID 12227038.
- Mohandas A, Vecchio D (2008). "A case report of Benzylpiperazine induced new onset affective symptoms in a patient with schizophrenia". European Psychiatry. 23: S315–S316. doi:10.1016/j.eurpsy.2008.01.1085. S2CID 145659495.
- Austin H, Monasterio E (2004). "Acute psychosis following ingestion of 'Rapture'". Australasian Psychiatry. 12 (4): 406–8. doi:10.1111/j.1440-1665.2004.02137.x. PMID 15715818.
- Gee P, Jerram T, Bowie D (March 2010). "Multiorgan failure from 1-benzylpiperazine ingestion—legal high or lethal high?". Clin Toxicol. 48 (3): 230–3. doi:10.3109/15563651003592948. PMID 20184432.
- Baselt, R. (2008). Disposition of Toxic Drugs and Chemicals in Man (8 ed.). Foster City, CA: Biomedical Publications. pp. 155–156. ISBN 978-0-931890-08-6.
- "Party On?". TV3 New Zealand. 9 April 2007. Archived from the original on 4 December 2007. Retrieved 14 April 2007.
- Arbo MD, Bastos ML, Carmo HF (May 2012). "Piperazine compounds as drugs of abuse". Drug and Alcohol Dependence. 122 (3): 174–85. doi:10.1016/j.drugalcdep.2011.10.007. PMID 22071119.
- Wilkins C, Girling M, Sweetsur P, Huckle T, Huakau J. "Legal party pill use in New Zealand: Prevalence of use, availability, health harms and 'gateway effects' of benzylpiperazine (BZP) and trifluorophenylmethylpiperazine (TFMPP)" (PDF). Centre for Social and Health Outcomes Research and Evaluation (SHORE). Archived from the original (PDF) on 18 March 2007. Retrieved 14 April 2007.
- Brennan K, Johnstone A, Fitzmaurice P, Lea R, Schenk S (2007). "Chronic benzylpiperazine (BZP) exposure produces behavioral sensitization and cross-sensitization to methamphetamine (MA)". Drug Alcohol Depend. 88 (2–3): 204–13. doi:10.1016/j.drugalcdep.2006.10.016. PMID 17125936.
- "Benzylpiperazine Legal Status". Erowid. 10 February 2015. Archived from the original on 21 December 2002. Retrieved 10 October 2021.
- "Warning on buying banned drug over web". The Australian. 10 October 2006. Archived from the original on 16 October 2007. Retrieved 14 April 2007.
- "Controlled Drugs and Substances Act (S.C. 1996, c. 19)". Canadian Department of Justice. 7 July 2014. Archived from the original on 15 December 2013. Retrieved 17 August 2014.
- "New drug under formal scrutiny: Council asks EMCDDA to assess risks of BZP". European Monitoring Centre for Drugs and Drug Addiction. 23 March 2007. Retrieved 14 April 2007.
- EUROPA (17 July 2007). "New Commission proposal to strengthen control of synthetic drug BZP". EU New Commission. Retrieved 1 February 2008.
- "New drug BZP to be placed under control across the EU" (PDF). European Monitoring Centre for Drugs and Drug Addiction. 3 March 2008. Retrieved 26 May 2008.
- "Misuse of Drugs (Classification of BZP) Amendment Bill" (PDF). New Zealand Parliament. 20 August 2007. Retrieved 26 May 2008.
- Sect. 8 of Medicines Act 1968 – Schedule 3, SI 3144 The Medicines for Human Use (Marketing Authorisations Etc) Regulations 1994
- "Thousands of 'pep' pills seized in Middlesbrough". Medicines and Healthcare products Regulatory Agency. 17 August 2006. Archived from the original on 28 September 2007. Retrieved 14 April 2007.
- "Move to outlaw two 'party' drugs". BBC News. 21 May 2009.
- "Summary of response: consultation on proposed control under the Misuse of Drugs Act 1971 of (1) 1-Benzylpiperazine and a group of substituted piperazines". Homeoffice.gov.uk. 30 October 2009. Archived from the original on 5 August 2012. Retrieved 1 January 2010.
- "Misuse of Drugs Act 1971 (Amendment) Order 2009".
- "BZP: Fast Facts". National Drug Intelligence Center. September 2004. Retrieved 22 April 2007.
- "DEA error on BZP potency" (PDF). Stargate International. 15 September 2004. Archived from the original (PDF) on 14 October 2008. Retrieved 22 April 2007.