Fat-Soluble Vitamins & Micronutrients: Vitamin D
In This Section
25-Hydroxyvitamin D
Background
Vitamin D (calciferol) comprises a group of fat soluble seco-sterols found naturally only in a few foods, such as fish-liver oils, fatty fish, mushrooms, egg yolks, and liver. The two major physiologically relevant forms of vitamin D are D2 (ergocalciferol) and D3 (cholecalciferol). Vitamin D3 is photosynthesized in the skin of vertebrates by the action of solar ultraviolet (UV) B radiation on 7-dehydrocholesterol (Fieser 1959). Vitamin D2 is produced by UV irradiation of ergosterol, which occurs in molds, yeast, and higher-order plants. Under conditions of regular sun exposure, dietary vitamin D intake is of minor importance. However, latitude, season, aging, sunscreen use, and skin pigmentation influence the production of vitamin D3 by the skin (Institute of Medicine 1997). Most of the dietary intake of vitamin D comes from fortified milk products and other fortified foods such as breakfast cereals and orange juice (Institute of Medicine 1997). Both vitamin D2 and D3 are used in nonprescription vitamin D supplements, but vitamin D2 is the form available by prescription in the United States (Holick 2007).
Vitamin D without a subscript represents either D2 or D3 or both and is biologically inert. Vitamin D from the skin or diet is only short-lived in circulation (with a half-life of 1–2 days), as it is either stored in fat cells or metabolized in the liver (Mawer 1972). In circulation, vitamin D is bound to vitamin D-binding protein and transported to the liver, where it is converted to 25-hydroxyvitamin D [25(OH)D] (DeLuca 1984). This major circulating form of vitamin D is a good reflection of cumulative effects of exposure to sunlight and dietary intake of vitamin D (Haddad 1973; Holick 1995) and is therefore used by clinicians to determine vitamin D status. To be biologically activated at physiologic concentrations, 25(OH)D must be converted in the kidneys to 1,25-dihydroxyvitamin D [1,25(OH)2D], which is thought to be responsible for most, if not all, of the biologic functions of vitamin D (DeLuca 1988; Reichel 1989). The production of 25(OH)D in the liver and of 1,25(OH)2D in the kidney is tightly regulated. In the liver, vitamin D-25-hydroxylase is down-regulated by vitamin D and its metabolites, thereby limiting any increase in the circulating concentration of 25(OH)D following intakes or following production of vitamin D after exposure to sunlight. In the kidney, in response to serum calcium and phosphorus concentrations, the production of 1,25(OH)2D is regulated through the action of parathyroid hormone (PTH) (DeLuca 1988; Reichel 1989).
Active vitamin D functions as a hormone, and its main biologic function in people is to maintain serum calcium and phosphorus concentrations within the normal range by enhancing the efficiency of the small intestine to absorb these minerals from the diet (DeLuca 1988; Reichel 1989). When dietary calcium intake is inadequate to satisfy the body's calcium requirement, 1,25(OH)2D, along with PTH, mobilizes calcium stores from the bone. In the kidney, 1,25(OH)2D increases calcium reabsorption by the distal renal tubules. Apart from these traditional calcium-related actions, 1,25(OH)2D and its synthetic analogs are increasingly recognized for their potent antiproliferative, prodifferentiative, and immunomodulatory activities (Nagpal 2005).
Vitamin D deficiency is characterized by inadequate mineralization or by demineralization of the skeleton. Among children, vitamin D deficiency is a common cause of bone deformities known as rickets. Vitamin D deficiency in adults leads to a mineralization defect in the skeleton, causing osteomalacia, and induces secondary hyperparathyroidism with consequent bone loss and osteoporosis. Potential roles for vitamin D beyond bone health, such as effects on muscle strength, the risk for cancer and for type 2 diabetes, are currently being studied. The Agency for Healthcare Research and Quality recently reviewed the effectiveness and safety of vitamin D on outcomes related to bone health (Cranney 2007). The report suggests that vitamin D supplementation has positive effects on bone health in postmenopausal women and older men.
Still, what constitutes the optimal intake of vitamin D remains a matter of some disagreement. Current recommendations from the Institute of Medicine (1997) call for 200 international units (IU) [5.0 micrograms (µg)] of vitamin D daily from birth through age 50, 400 IU (10 µg) for those aged 51–70 years, and 600 IU (15 µg) for those older than 70 years. According to the Dietary Guidelines for Americans (U.S. Department of Health and Human Services and U.S. Department of Agriculture 2005) older adults, people with dark skin, and people exposed to insufficient UV B radiation should consume extra vitamin D from vitamin D-fortified foods or supplements. The American Cancer Society Guidelines on Nutrition and Physical Activity for Cancer Prevention echo this recommendation (Kushi 2006). Some experts say that optimal amounts for all adults are closer to 800–1000 IU (20–25 µg) daily (Vieth 2007; Bischoff-Ferrari 2006; Dawson-Hughes 2005). The tolerable upper intake level for vitamin D is 2000 IU (50 µg) per day in North America and in Europe; however, some scientists are calling for an upward revision (Hathcock 2007; Vieth 2006).
Some clinical laboratories use conventional units for 25(OH)D (nanogram per milliliter [ng/mL]) whereas other laboratories use international system (SI) units (nanomole per liter [nmol/L]). The conversion factor to SI units is: 1 ng/mL = 2.496 nmol/L.
No common definition exists for adequate vitamin D status measured as 25(OH)D serum concentrations (Dawson-Hughes 2005). The Institute of Medicine (1997) defined vitamin D deficiency as serum 25(OH)D concentrations of less than 11 ng/mL (27.5 nmol/L) for neonates, infants, and young children. Because the lower limit of the normal range can be as low as 8 ng/mL (20 nmol/L) and as high as 15 ng/mL (37.5 nmol/L), depending on the geographic location, vitamin D deficiency has been defined as a concentration of less than 12 ng/mL (mid-range between 8 and 15 ng/mL) for adults (Institute of Medicine 1997). More recently, some scientists have suggested that the criteria used to define adequate status should be revised upwards; serum 25(OH)D concentrations between 20 ng/mL (50 nmol/L) and 32 ng/mL (80 nmol/L) have been defined as sufficient (Hollis 2005; Dawson-Hughes 2005; Bischoff-Ferrari 2006; Norman 2007). A common definition for high serum vitamin D concentrations is also lacking. The Institute of Medicine (1997) used serum calcium concentrations greater than 11 milligrams per deciliter (mg/dL) for assessing the potential for increased risk of harm associated with high vitamin D intakes. To date, however, no evidence has surfaced of adverse effects with serum 25(OH)D concentrations as high as 56 ng/mL (140 nmol/L) in healthy individuals (Vieth 1999).

Different assays measure serum 25(OH)D, and at times large variations occur among methods and even between laboratories using the same method (Singh 2008; Binkley 2004; Carter 2004). Standard reference materials (SRMs) for serum 25(OH)D are currently under development by the U.S. National Institute of Standards and Technology (U.S. NIST). Improvement in the agreement between laboratories and methods is expected as laboratories begin to use the SRMs.
More information on vitamin D is available online:
- American Society for Nutrition fact sheet*
- Institute of Medicine Dietary Reference Intake report*
- National Institutes of Health fact sheet*
Since 1988, NHANES has monitored the vitamin D status of the U.S. population. By design, this survey collects information and biological samples in the summer from people living at higher latitudes and in the winter from people living at lower latitudes. Because the different racial and ethnic groups are not evenly distributed across all geographic regions in the United States, the season-latitude structure of the survey can affect comparisons by race or ethnicity. In two seasonal subpopulations from NHANES III (1988–1994), Looker et al. (2002) showed that in the winter and lower latitude subpopulation, 1–5 percent and 25–57 percent had 25(OH)D concentrations less than 10 ng/mL (25 nmol/L) and less than 25 ng/mL (62.5 nmol/L), respectively. In the summer and higher latitude subpopulation, 1–3 percent and 21–49 percent had 25(OH)D concentrations below these cutoffs. Mean 25(OH)D concentrations were highest in non-Hispanic whites, intermediate in Mexican Americans, and lowest in non-Hispanic blacks. Nesby-O'Dell et al. (2002) restricted the analysis of NHANES III data to African-American and white women of reproductive age and found the prevalence of hypovitaminosis [25(OH)D concentrations < 15 ng/mL (37.5 nmol/L)] to be 42.2 percent among African Americans and 4.2 percent among whites.
Selected Observations and Highlights
The following sample observations and figures are taken from the tables of 2001–2002 data contained in this report. Statements about categorical differences between demographic groups noted below are based on non-overlapping confidence limits from univariate analysis without adjusting for demographic variables (e.g., age, sex, race/ethnicity) or other determinants of these blood concentrations (e.g., dietary intake, supplement usage, smoking, BMI). A multivariate analysis may alter the size and statistical significance of these categorical differences. Furthermore, additional significant differences of smaller magnitude may be present despite their lack of mention here (e.g., if confidence limits slightly overlap or if differences are unobservable before covariate adjustment has occurred). For a selection of citations of descriptive NHANES papers related to these biochemical indicators of diet and nutrition, see Appendix E.
General Observations
- Serum 25(OH)D concentrations are similar throughout all age groups, except that children 6–11 years of age have higher concentrations than do people in other age groups.
- Non-Hispanic whites have higher concentrations of 25(OH)D than do Mexican Americans, who themselves have higher concentrations than do non-Hispanic blacks.
- Approximately 10 percent of the population has concentrations of 25(OH)D that are less than 11 ng/mL. The values at the 10th percentile vary greatly by racial-ethnic group, with non-Hispanic blacks having the highest prevalence of low 25(OH)D concentrations.
Highlights
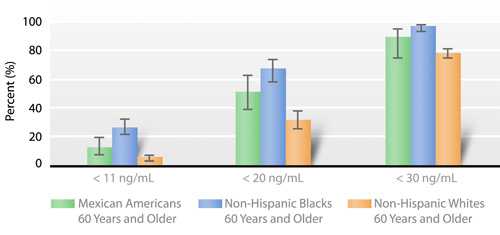
Because of the current disagreement regarding appropriate criteria by which to define adequate status on the basis of serum 25(OH)D concentrations, the figure below presents prevalence estimates for older people (≥ 60 years) for three cut-off values: 11 ng/mL, 20 ng/mL, and 30 ng/mL. Regardless of the cut-off value, non-Hispanic blacks have the highest prevalence of low serum 25(OH)D concentrations (Fig. 2.a).
Tables
Serum 25-hydroxyvitamin D
- Table 2.9.a. Serum 25-hydroxyvitamin D: Total population
- Table 2.9.b. Serum 25-hydroxyvitamin D: Mexican Americans
- Table 2.9.c. Serum 25-hydroxyvitamin D: Non-Hispanic blacks
- Table 2.9.d. Serum 25-hydroxyvitamin D: Non-Hispanic whites
References
Binkley N, Krueger D, Cowgill CS, Plum L, Lake E, Hansen KE, et al. Assay variation confounds the diagnosis of hypovitaminosis D: a call for standardization. J Clin Endocrinol Metab. 2004;89:3152-7.
Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18-28.
Carter GD, Carter R, Jones J, Berry J. How accurate are assays for 25-hydroxyvitamin D? Data from the international vitamin D external quality assessment scheme. Clin Chem. 2004;50:2195-7.
Cranney A, Horsley T, O'Donnell S, Weiler HA, Puil L, Ooi DS, et al. Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess (Full Rep). 2007;158:1-235.
Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16:713-6.
DeLuca HF. The metabolism, physiology, and function of vitamin D. In: Kumar R, editor. Vitamin D: basic and clinical aspects. Boston (MA): M. Nijhoff Publishers; 1984. p. 1-68.
DeLuca HF. The vitamin D story: a collaborative effort of basic science and clinical medicine. FASEB J. 1988;2:224-36.
Fieser LF, Fieser M. Vitamin D. In: Steroids. 1st ed. New York: Reinhold Publishing Corporation;1959. p. 90-168.
Haddad JG, Hahn TJ. Natural and synthetic sources of circulating 25-hydroxy-vitamin D in man. Nature. 1973;244:515-7.
Hathcock JN, Shao A, Vieth R, Heaney R. Risk assessment for vitamin D. Am J Clin Nutr. 2007;85:6-18.
Holick MF. Vitamin D: photobiology, metabolism, and clinical applications. In: DeGroot LJ, editor. Endocrinology, Vol 2, 3rd ed. Philadelphia (PA): WB Saunders; 1995. p. 990-1013.
Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-81.
Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr. 2005;135:317-22.
Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes: calcium, phosphorus, magnesium, vitamin D and fluoride. Washington, D.C.: National Academy Press; 1997.
Kushi LH, Byers T, Doyle C, Bandera EV, McCullough M, Gansler T, et al. American Cancer Society guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2006;56:254-81.
Looker AC, Dawson-Hughs B, Calvo MS, Gunter EW, Sayhoun NR. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30:771-7.
Mawer EB, Blackhouse J, Holman CA, Lumb GA, Stanbury DW. The distribution and storage of vitamin D and its metabolites in human tissues. Clin Sci. 1972;43:413-31.
Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev. 2005;26:662-87.
Nesby-O'Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, et al. Hypovitaminosis D and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;76:187-92.
Norman AW, Bouillon R, Whiting SJ, Vieth R, Lips P. 13th Workshop consensus for vitamin D nutritional guidelines. J Steroid Biochem Mol Biol. 2007;103:204-5.
Reichel H, Koeffler HP, Norman AW. The role of vitamin D endocrine system in health and disease. N Engl J Med. 1989;320:980-91.
Singh RJ. Are clinical laboratories prepared for accurate testing of 25-hydroxy vitamin D? Clin Chem. 2008;54:221-2.
U.S. Department of Health and Human Services and U.S. Department of Agriculture. Dietary guidelines for Americans, 2005. 6th ed. Washington, D.C.: U.S. Government Printing Office; January 2005.
Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr. 1999;69:842-56.
Vieth R. Critique of the considerations for establishing the tolerable upper intake level for vitamin D: critical need for revision upwards. J Nutr. 2006;136:1117-22.
Vieth R. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr. 2007;85:649-50.
Related Links
- American Society for Nutrition
Vitamin D Fact Sheet*
- Centers for Disease Control and Prevention
Health Topic: Breastfeeding and Vitamin D Supplementation
- Institute of Medicine Dietary Reference Intakes
Vitamin D Report*
- National Institutes of Health, Office of Dietary Supplements
Vitamin D Fact Sheet
* Links to non-Federal organizations found at this site are provided solely as a service to our users. These links do not constitute an endorsement of these organizations or their programs by CDC or the Federal Government, and none should be inferred. CDC is not responsible for the content of the individual organization Web pages found at these links.
Top of Page- Page last reviewed: July 30, 2008
- Page last updated: July 30, 2008
- Content source:


 ShareCompartir
ShareCompartir