Bone health
The human skeletal system is a complex organ in constant equilibrium with the rest of the body. In addition to support and structure of the body, bone is the major reservoir for many minerals and compounds essential for maintaining a healthy pH balance.[1] The deterioration of the body with age renders the elderly particularly susceptible to and affected by poor bone health. Illnesses like osteoporosis, characterized by weakening of the bone's structural matrix, increases the risk of hip-fractures and other life-changing secondary symptoms. In 2010, over 258,000 people aged 65 and older were admitted to the hospital for hip fractures.[2] Incidence of hip fractures is expected to rise by 12% in America, with a projected 289,000 admissions in the year 2030.[3] Other sources estimate up to 1.5 million Americans will have an osteoporotic-related fracture each year.[4] The cost of treating these people is also enormous, in 1991 Medicare spent an estimated $2.9 billion for treatment and out-patient care of hip fractures, this number can only be expected to rise.[5]
Amino acid metabolism
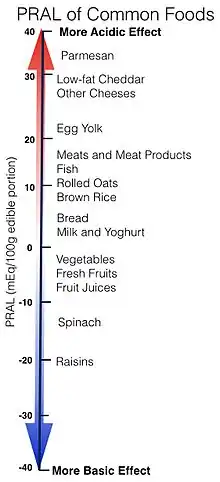
When more sulfur containing amino acids, methionine and cystine, are consumed than the body can use for growth and repair, they are broken down yielding sulfate, or sulfuric acid among other products. Animal foods such as meat, dairy, and eggs are high in protein and “dietary animal protein intake is highly correlated with renal net acid excretion”.[6] Research dating back to the early 1900s has shown correlations between high protein diets and increased acid excretion.[7] One measure of the acidic or basic effects foods have in the body is Potential Renal Acid Load (PRAL). Cheeses with protein content of 15 g protein/100g or higher have a high PRAL value of 23.6 mEq/100 g edible portion. Meats, fish, other cheeses and flour or noodles all have a PRAL around 8.0 mEq/100 g edible portion, where fruits and vegetables actually have a negative PRAL.[1][8]

In healthy adults, bone is undergoing constant repair and renewal. New bone is deposited by osteoblast cells and resorbed or destroyed by osteoclast cells. This addition and subtraction of bone usually yields no net change in the overall mass of the skeleton, but the turnover process can be significantly affected by pH.[1]
Bone mineral density
Bone mineral density (BMD) is a measure commonly used to quantify bone health. A lower BMD value indicates an increased risk of an osteoporosis or a fracture.[9] There is a large range of factors influencing BMD. Protein consumption has shown to be beneficial for bone density by providing amino acid substrates necessary for bone matrix formation. It is also thought that blood concentration of the bone formation stimulant, Insulin-like Growth Factor-I (IGF-I), is increased from high protein consumption and parathyroid hormone (PTH), a bone resorption stimulant, is decreased.[10] Although protein has shown to be beneficial for increasing bone mass, or bone mineral density, there is no significant association between protein intake and fracture incidence.[11] In other words, a low BMD can be predictive of osteoporosis and increased fracture risk, but a higher BMD does not necessarily mean better bone health. High BMD is also correlated with other health issues.[12] For example, a higher BMD has also been associated with increased risk of breast cancer.[13]
Acid–base homeostasis
Most metabolic processes have a specific and narrow range of pH where operation is possible, multiple regulatory systems are in place to maintain homeostasis. Fluctuations away from optimal operating pH can slow or impair reactions and possibly cause damage to cellular structures or proteins. To maintain homeostasis the body may excrete excess acid or base through the urine, via gas exchange in the lungs, or buffer it in the blood.[14] The bicarbonate buffering system of blood plasma effectively holds a steady pH and helps to hold extracellular pH around 7.35.[15] The kidneys are responsible for the majority of acid-base regulation but can excrete urine no lower than a pH of 5. This means that a 330mL can of cola, for example, usually ranging in pH from 2.8–3.2, would need to be diluted 100 fold before being excreted. Instead of producing 33L of urine from one can of cola, the body relies on buffer to neutralize the acid.[1] Systemic acidosis can be the result of multiple factors, not just diet. Anaerobic exercise, diabetes, AIDS, aging, menopause, inflammation, infections, tumours, and other wounds and fractures all contribute to acidosis. Blood has an average pH of 7.40 but interstitial fluid can vary. Interstitial pH of the skin, for example, is ~7.1. There is no data available for bone.[16]
Homocysteine
Homocysteine, a non-protein amino acid and analogue to the protein amino acid cystine, has been shown to have negative effects on bone health. Higher homocysteine concentrations are likely a result of folate, vitamin B12 B6 deficiencies. In addition, it was found that homocysteine concentration was significantly affected by physical activity. The stimulation of the skeleton through physical activity promotes positive bone remodelling and decreases levels of homocysteine, independently from nutritional intake. Four methods have been proposed regarding the interaction of homocysteine and bone; increase in osteoclast activity, decrease in osteoblast activity, decrease in bone blood flow, and direct action of homocysteine on bone matrix. Homocysteine inhibits lysyl oxidase which is responsible for post-translational modifications of collagen, a key component to bone structure[17]
Osteoclast cells
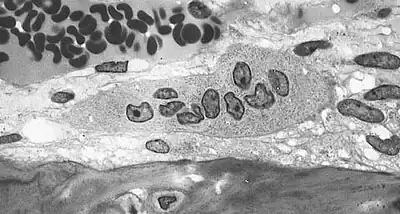
Osteoclasts are located on the surface of bones and form resorption pits by excreting H+ to the bone surface removing hydroxyapatite, multiple bone minerals, and organic components: collagen and dentin. The purpose of bone resorption is to release calcium to the blood stream for various life processes.[17] These resorption pits are visible under electron microscopy and distinctive trails are formed from prolonged resorption. Osteoclasts have shown to be “absolutely dependent on extracellular acidification”.[14] A drop in pH of <0.1 units can cause a 100% increase in osteoclast cell activity, this effect persists with prolonged acidosis with no desensitization, “amplifying the effects of modest pH differences”. Osteoclast cells show little or no activity at pH 7.4 and are most active at pH 6.8 but can be further stimulated by other factors such as parathyroid hormone.[16]
Osteoblast cells

Osteoblast are responsible for the mineralization and construction of bone matrix. They are responsible for the formation or production of bone tissue. [18] The origin of the osteoblasts and osteoclasts is from primitive precursor cells found in bone marrow. [18]Like osteoclast cells, osteoblast cell activity is directly related to extracellular pH mirroring of osteoclast activity. At pH 7.4, where osteoclasts are inactive, osteoblast are at peak activity. Likewise, at pH 6.9 osteoblast activity is non-existent.[16] The hormone estrogen is also important for osteoblast regulation. In postmenopausal women estrogen levels are decreased which has negative effects on bone remodeling. Homocysteine further exacerbates this problem by reducing estrogen receptor α mRNA transcription. Thus reducing any beneficial effect that estrogen plays on bone remodeling.[17]
Bone balance
Acidosis inhibits bone osteoblast matrix mineralization with reciprocal effect on osteoclast activation. The combined responses of these cells to acidosis maximizes the availability of hydroxyl ions in solution that can be used to buffer protons.[16] The utilization of bone to buffer even a small percentage of daily acid production can lead to significant loss of bone mass in the course of a decade.[6] Additionally, as the body ages there is a steady decline in renal function. Metabolic acidosis can become more severe as kidney function weakens, and the body will depend more heavily on bone and blood to maintain acid-base homeostasis.[10]
Diet
There is no one food or nutrient capable of providing adequate bone health on its own. Instead, a balanced diet sufficient in fruits and vegetables for their vitamins, minerals, and alkalinizing substrates is thought to be most beneficial. High protein diets supply larger amounts of amino acids that could be degraded to acidic compounds. Protein consumption above the Recommended Dietary Allowance is also known to be beneficial to calcium utilization. Overall it is understood that high-protein diets have a net benefit for bone health because changes in IGF-I and PTH concentrations outweigh the negative effects of metabolic acid production.[10] The source of protein, plant or animal, does not matter in terms of acid produced from amino acid metabolism. Any differences in methionine and cysteine content is not significant to affect the overall potential renal acid load (PRAL) of the food. In addition to their acid precursor protein content, plants also contain significant amounts of base precursors. Potassium bicarbonate, a basic salt, is produced via the metabolism of other organic potassium salts: citrate, malate, and gluconate, which are substantial in plants. The discrepancy observed in PRAL is accounted for by differences in base precursor content.[6][8]
See also
References
- 1 2 3 4 Barzel, US; Massey, LK (June 1998). "Excess dietary protein can adversely affect bone". The Journal of Nutrition. 128 (6): 1051–53. doi:10.1093/jn/128.6.1051. PMID 9614169.
- ↑ "National Hospital Discharge Survey (NHDS)". National Center for Health Statistics. Archived from the original on 30 November 2013. Retrieved 24 November 2013.
- ↑ Stevens, JA; Rudd, RA (October 2013). "The impact of decreasing U.S. hip fracture rates on future hip fracture estimates". Osteoporosis International. 24 (10): 2725–28. doi:10.1007/s00198-013-2375-9. PMC 4717482. PMID 23632827.
- ↑ Hyson, DA (September 2011). "A comprehensive review of apples and apple components and their relationship to human health". Advances in Nutrition. 2 (5): 408–20. doi:10.3945/an.111.000513. PMC 3183591. PMID 22332082.
- ↑ "Incidence and costs to Medicare of fractures among Medicare beneficiaries aged >65 years – United States, July 1991–June 1992". Centers for Disease Control and Prevention. MMWR. 45 (41): 877–83. 1996. PMID 8927007.
- 1 2 3 Sellmeyer, DE; Stone, KL; Sebastian, A; Cummings, SR (January 2001). "A high ratio of dietary animal to vegetable protein increases the rate of bone loss and the risk of fracture in postmenopausal women. Study of Osteoporotic Fractures Research Group". The American Journal of Clinical Nutrition. 73 (1): 118–22. doi:10.1093/ajcn/73.1.118. PMID 11124760.
- ↑ Sherman, H. C.; Gettler, A. O. (1 May 1911). "The balance of acid-forming and base-forming elements in foods, and its relation to ammonia metabolism". Experimental Biology and Medicine. 8 (5): 119–20. doi:10.3181/00379727-8-71. S2CID 81933816.
- 1 2 Remer, T; Manz, F (July 1995). "Potential renal acid load of foods and its influence on urine pH". Journal of the American Dietetic Association. 95 (7): 791–97. doi:10.1016/S0002-8223(95)00219-7. PMID 7797810.
- ↑ Lips, P (Aug 18, 1997). "Epidemiology and predictors of fractures associated with osteoporosis". The American Journal of Medicine. 103 (2A): 3S–8S, discussion 8S–11S. doi:10.1016/s0002-9343(97)90021-8. PMID 9302892.
- 1 2 3 Cao, JJ; Nielsen, FH (November 2010). "Acid diet (high-meat protein) effects on calcium metabolism and bone health". Current Opinion in Clinical Nutrition and Metabolic Care. 13 (6): 698–702. doi:10.1097/MCO.0b013e32833df691. PMID 20717017. S2CID 1332501.
- ↑ Kerstetter, JE (December 2009). "Dietary protein and bone: a new approach to an old question". The American Journal of Clinical Nutrition. 90 (6): 1451–52. doi:10.3945/ajcn.2009.28812. PMID 19864406.
- ↑ Gregson, CL; Hardcastle, SA; Cooper, C; Tobias, JH (June 2013). "Friend or foe: high bone mineral density on routine bone density scanning, a review of causes and management". Rheumatology (Oxford, England). 52 (6): 968–85. doi:10.1093/rheumatology/ket007. PMC 3651616. PMID 23445662.
- ↑ Lucas, FL; Cauley, JA; Stone, RA; Cummings, SR; Vogt, MT; Weissfeld, JL; Kuller, LH (Jul 1, 1998). "Bone mineral density and risk of breast cancer: differences by family history of breast cancer. Study of Osteoporotic Fractures Research Group". American Journal of Epidemiology. 148 (1): 22–29. doi:10.1093/oxfordjournals.aje.a009554. PMID 9663400.
- 1 2 Arnett, T (May 2003). "Regulation of bone cell function by acid-base balance". The Proceedings of the Nutrition Society. 62 (2): 511–20. doi:10.1079/pns2003268. PMID 14506899.
- ↑ Bonjour, JP (October 2013). "Nutritional disturbance in acid-base balance and osteoporosis: a hypothesis that disregards the essential homeostatic role of the kidney". The British Journal of Nutrition. 110 (7): 1168–77. doi:10.1017/S0007114513000962. PMC 3828631. PMID 23551968.
- 1 2 3 4 Arnett, TR (February 2008). "Extracellular pH regulates bone cell function". The Journal of Nutrition. 138 (2): 415S–18S. doi:10.1093/jn/138.2.415S. PMID 18203913.
- 1 2 3 Vacek, TP; Kalani, A; Voor, MJ; Tyagi, SC; Tyagi, N (Mar 1, 2013). "The role of homocysteine in bone remodeling". Clinical Chemistry and Laboratory Medicine. 51 (3): 579–90. doi:10.1515/cclm-2012-0605. PMC 3951268. PMID 23449525.
- 1 2 Krause, Marie V.; Raymond, Janice L. (2008). Krause's Food & Nutrition Therapy. Saunders/Elsevier. ISBN 978-1-4160-3401-8.
External links
- PRAL Calculator Calculate PRAL per meal, recipe or day's total from nutrition data.