Catechol-O-methyltransferase inhibitor
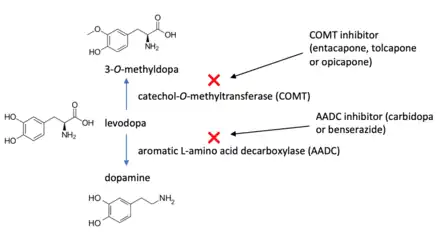
A catechol-O-methyltransferase (COMT) inhibitor is a drug that inhibits the enzyme catechol-O-methyltransferase. This enzyme methylates catecholamines such as dopamine, norepinephrine and epinephrine. It also methylates levodopa. COMT inhibitors are indicated for the treatment of Parkinson's disease in combination with levodopa and an aromatic L-amino acid decarboxylase inhibitor (e.g. carbidopa or benserazide). The therapeutic benefit of using a COMT inhibitor is based on its ability to prevent the methylation of levodopa to 3-O-methyldopa, thus increasing the bioavailability of levodopa. COMT inhibitors significantly decrease off time in people with Parkinson's disease also taking carbidopa/levodopa.[1]
List of COMT inhibitors
- entacapone (Comtan, Comtess, Stalevo)
- nebicapone
- nitecapone
- opicapone (Ongentys)
- tolcapone (Tasmar)
Entacapone and opicapone are peripheral inhibitors, unable to cross the blood-brain barrier. Tolcapone is able to cross the blood-brain barrier.[2] Tolcapone has been associated with at least three fatal cases of acute liver failure and is thus only rarely prescribed.[3] Patients taking tolcapone must be monitored for hepatic failure. Entacapone and opicapone have not been associated with hepatotoxicity.[4][5]
Adverse effects
- nausea
- orthostatic hypotension
- vivid dreams
- confusion
- hallucinations
- hepatotoxicity (only tolcapone)
- diarrhea
- drowsiness
- urine discoloration
- dyskinesia
See also
- Medication Management of Parkinson's disease
- catechol-O-methyltransferase
- entacapone
- carbidopa/levodopa/entacapone
- tolcapone
- opicapone
- nitecapone
References
- ↑ "Entacapone improves motor fluctuations in levodopa-treated Parkinson's disease patients. Parkinson Study Group". Annals of Neurology. 42 (5): 747–755. Nov 1997. doi:10.1002/ana.410420511. ISSN 0364-5134. PMID 9392574. S2CID 975995.
- ↑ Lang, Anthony E.; Connolly, Barbara S. (2014-04-23). "Pharmacological Treatment of Parkinson Disease: A Review". JAMA. 311 (16): 1670–1683. doi:10.1001/jama.2014.3654. ISSN 0098-7484. PMID 24756517.
- ↑ Olanow, C. Warren; Watkins, Paul B. (Sep 2007). "Tolcapone: an efficacy and safety review". Clinical Neuropharmacology. 30 (5): 287–294. doi:10.1097/wnf.0b013e318038d2b6. ISSN 0362-5664. PMID 17909307. S2CID 19148461.
- ↑ Scott, Lesley J. (2016-08-06). "Opicapone: A Review in Parkinson's Disease". Drugs. 76 (13): 1293–1300. doi:10.1007/s40265-016-0623-y. ISSN 0012-6667. PMID 27498199. S2CID 5787752.
- ↑ Watkins, P (2000). "COMT inhibitors and liver toxicity". Neurology. 55 (11 Suppl 4): S51-2. PMID 11147510.
- ↑ Govindasamy, Hunday; Magudeeswaran, Sivanandam; Poomani, Kumaradhas (2020-12-11). "Identification of novel flavonoid inhibitor of Catechol-O-Methyltransferase enzyme by molecular screening, quantum mechanics/molecular mechanics and molecular dynamics simulations". Journal of Biomolecular Structure and Dynamics. 38 (18): 5307–5319. doi:10.1080/07391102.2019.1699446. ISSN 0739-1102. PMID 31779524. S2CID 208356889.